
Which of the following is a ring chain of i-butene?
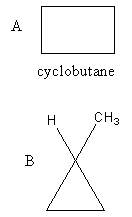
C. Both A and B
D. none of these

Answer
563.4k+ views
Hint:The molecules having the same molecular formula but a different arrangement of atoms are known as isomers. We will determine the molecular formula of each given compound to determine whether it is an isomer of i-butene or not. To determine the molecular formula we will count the number of each type of atom.
Complete solution:
The structure of i-butene is as follows:
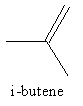
i-butene has four carbon atoms and eight hydrogen atoms. So, the molecular formula of i-butene is ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}$ .
As we knew the isomers have same molecular formula so, we will determine the molecular formula of compound A and B as follows:
The structure of the given compounds A and B is as follows:
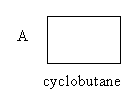
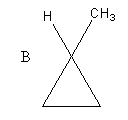
Compound A has four carbon atoms and eight hydrogen atoms. So, the molecular formula of compound A, cyclobutane is ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}$ .
Compound B also has four carbon atoms and eight hydrogen atoms. So, the molecular formula of compound B, is ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}$ .
So, as the molecular formula of compound A and B is the same as of i-butene so, compound A and B are the isomers of i-butene.
So, A and B both are the ring chain of i-butene.
Therefore, the correct answer is (A).
Note:Isomers have the same molecular formula but different chemical formula. A molecular formula shows the total number of an atom in the compound. The chemical formula shows the different groups of atoms of a molecule. To draw the structure of the isomers from the given molecular formula first we determine the degree of unsaturation by using the following formula:
Complete solution:
The structure of i-butene is as follows:

i-butene has four carbon atoms and eight hydrogen atoms. So, the molecular formula of i-butene is ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}$ .
As we knew the isomers have same molecular formula so, we will determine the molecular formula of compound A and B as follows:
The structure of the given compounds A and B is as follows:


Compound A has four carbon atoms and eight hydrogen atoms. So, the molecular formula of compound A, cyclobutane is ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}$ .
Compound B also has four carbon atoms and eight hydrogen atoms. So, the molecular formula of compound B, is ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}$ .
So, as the molecular formula of compound A and B is the same as of i-butene so, compound A and B are the isomers of i-butene.
So, A and B both are the ring chain of i-butene.
Therefore, the correct answer is (A).
Note:Isomers have the same molecular formula but different chemical formula. A molecular formula shows the total number of an atom in the compound. The chemical formula shows the different groups of atoms of a molecule. To draw the structure of the isomers from the given molecular formula first we determine the degree of unsaturation by using the following formula:
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




