
Which of the following hybrids is electron-precise hydride?
A. ${{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}$
B. ${\text{N}}{{\text{H}}_{\text{3}}}$
C. ${{\text{H}}_{\text{2}}}{\text{O}}$
D. ${\text{C}}{{\text{H}}_{\text{4}}}$
Answer
561.6k+ views
Hint: To answer this question we should know what electron-precise hydride means. The hydrides mean the compound will have hydrogen atoms in the form of an anion. Now come to the electron-precise, it means that an atom that is forming bonds with hydrogen atoms should have the exact number of electrons as it requires to form the normal covalent bonds.
Complete answer:
Electron-precise hybrids contain the exact number of electrons to form a normal covalent bond means the electron number in its valence shell will be equal to the number of covalent bonds formed by it.
We will draw the geometry of each given molecule to determine the electron-precise hydride. The hydride which will have valence electrons equal to the number of the covalent bond will be the electron-precise hydride.
Let us discuss the structure of molecules one by one.
${{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}$ is a dimer of ${\text{B}}{{\text{H}}_{\text{3}}}$ which is generally known as diborane. The valence electronic configuration of boron is ${\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{1}}}$. The valence shell of the boron has only three electrons. It requires five electrons to complete its octet. Boron cannot form five bonds, so it ${\text{B}}{{\text{H}}_{\text{3}}}$forms a dimer to complete its octet.
The structure of the dimer is as follows:
Diborane is electron deficient because each boron has a total of six electrons in four bonds. In this molecule, each boron atom is bonded to 2 terminal hydrogen atoms each also, two boron atoms are held together by two hydrogen atoms in bridging as shown below:
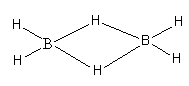
So, as each boron has three valence electrons and forms four covalent bonds, so diborane is electron-precise hydride.
${\text{N}}{{\text{H}}_{\text{3}}}$, The valence electronic configuration of nitrogen is ${\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^3}$. N – atom has 5 valence electrons, out of which 3 electrons form covalent bonds with H-atoms and two electrons remain unbonded. These electrons are called lone pairs of electrons.
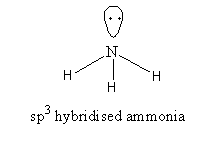
It has a trigonal pyramidal structure. The shape is predicted by valence-shell pair repulsion theory [VSEPR]. The bond angle is ${\text{106}}{\text{.}}{{\text{7}}^{\text{o}}}$.
o, the nitrogen has five valence electrons but it forms three covalent bonds. All electrons are not used for bond formation. It has an electron-rich center therefore ${\text{N}}{{\text{H}}_{\text{3}}}$is not an electron-precise hydride.
${{\text{H}}_{\text{2}}}{\text{O}}$, The valence electronic configuration of oxygen is ${\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^4}$. Oxygen atom has 6 valence electrons of which two electrons form a bond with two hydrogen atoms but 4 electrons [two pairs] remain unbonded.
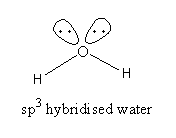
Water has ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridization but due to the presence of two lone pair of electrons ${\text{H}} - {\text{O}} - {\text{H}}$ angle is ${\text{104}}{\text{.}}{{\text{5}}^{\text{o}}}$ .
So, the oxygen has six valence electrons but it forms two covalent bonds. All electrons are not used for bond formation. It has an electron-rich centre therefore water is not an electron-precise hydride.
${\text{C}}{{\text{H}}_{\text{4}}}$, The valence electronic configuration of carbon is ${\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{2}}}$. Carbon atoms have 4 valence electrons. It forms four bonds with four hydrogen atoms.
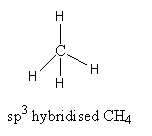
It has tetrahedral geometry with bond angle ${\text{109}}{\text{.2}}{{\text{8}}^{\text{o}}}$. So, the carbon has four valence electrons and forms four covalent bonds. All electrons are used for bond formation. Therefore, ${\text{C}}{{\text{H}}_{\text{4}}}$ is electron-precise hydride.
Therefore, option (D) ${\text{C}}{{\text{H}}_{\text{4}}}$ is the correct answer.
Note:Molecules are either electron-deficient or electron-rich or neutral. If all valence electrons are used to form a covalent bond, then the molecule is precise. The electron-precise molecules have perfect or we can say regular geometry or shape. Such as the geometry of ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridization is tetrahedral which is of electron-precise molecule methane. The non-electron-precise molecule has a different geometry and shape. Such as the geometry of ammonia and water is tetrahedral but the shape is pyramidal and bent respectively.
Complete answer:
Electron-precise hybrids contain the exact number of electrons to form a normal covalent bond means the electron number in its valence shell will be equal to the number of covalent bonds formed by it.
We will draw the geometry of each given molecule to determine the electron-precise hydride. The hydride which will have valence electrons equal to the number of the covalent bond will be the electron-precise hydride.
Let us discuss the structure of molecules one by one.
${{\text{B}}_{\text{2}}}{{\text{H}}_{\text{6}}}$ is a dimer of ${\text{B}}{{\text{H}}_{\text{3}}}$ which is generally known as diborane. The valence electronic configuration of boron is ${\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{1}}}$. The valence shell of the boron has only three electrons. It requires five electrons to complete its octet. Boron cannot form five bonds, so it ${\text{B}}{{\text{H}}_{\text{3}}}$forms a dimer to complete its octet.
The structure of the dimer is as follows:
Diborane is electron deficient because each boron has a total of six electrons in four bonds. In this molecule, each boron atom is bonded to 2 terminal hydrogen atoms each also, two boron atoms are held together by two hydrogen atoms in bridging as shown below:

So, as each boron has three valence electrons and forms four covalent bonds, so diborane is electron-precise hydride.
${\text{N}}{{\text{H}}_{\text{3}}}$, The valence electronic configuration of nitrogen is ${\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^3}$. N – atom has 5 valence electrons, out of which 3 electrons form covalent bonds with H-atoms and two electrons remain unbonded. These electrons are called lone pairs of electrons.

It has a trigonal pyramidal structure. The shape is predicted by valence-shell pair repulsion theory [VSEPR]. The bond angle is ${\text{106}}{\text{.}}{{\text{7}}^{\text{o}}}$.
o, the nitrogen has five valence electrons but it forms three covalent bonds. All electrons are not used for bond formation. It has an electron-rich center therefore ${\text{N}}{{\text{H}}_{\text{3}}}$is not an electron-precise hydride.
${{\text{H}}_{\text{2}}}{\text{O}}$, The valence electronic configuration of oxygen is ${\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^4}$. Oxygen atom has 6 valence electrons of which two electrons form a bond with two hydrogen atoms but 4 electrons [two pairs] remain unbonded.

Water has ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridization but due to the presence of two lone pair of electrons ${\text{H}} - {\text{O}} - {\text{H}}$ angle is ${\text{104}}{\text{.}}{{\text{5}}^{\text{o}}}$ .
So, the oxygen has six valence electrons but it forms two covalent bonds. All electrons are not used for bond formation. It has an electron-rich centre therefore water is not an electron-precise hydride.
${\text{C}}{{\text{H}}_{\text{4}}}$, The valence electronic configuration of carbon is ${\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{2}}}$. Carbon atoms have 4 valence electrons. It forms four bonds with four hydrogen atoms.

It has tetrahedral geometry with bond angle ${\text{109}}{\text{.2}}{{\text{8}}^{\text{o}}}$. So, the carbon has four valence electrons and forms four covalent bonds. All electrons are used for bond formation. Therefore, ${\text{C}}{{\text{H}}_{\text{4}}}$ is electron-precise hydride.
Therefore, option (D) ${\text{C}}{{\text{H}}_{\text{4}}}$ is the correct answer.
Note:Molecules are either electron-deficient or electron-rich or neutral. If all valence electrons are used to form a covalent bond, then the molecule is precise. The electron-precise molecules have perfect or we can say regular geometry or shape. Such as the geometry of ${\text{s}}{{\text{p}}^{\text{3}}}$ hybridization is tetrahedral which is of electron-precise molecule methane. The non-electron-precise molecule has a different geometry and shape. Such as the geometry of ammonia and water is tetrahedral but the shape is pyramidal and bent respectively.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




