
Which of the following have delocalised electrons?
A.Benzene
B.Cyclohexane
C.\[C{H_4}\]
D.\[{C_2}{H_6}\]
Answer
573.9k+ views
Hint: We have to remember that a pair of electrons which don’t make any bond are known as lone pairs whereas a pair of electrons, which is found in the paired condition they are known as electron pairs. Lone pair electron can move within the molecule.
Complete answer:
We must remember that the delocalised electrons are those electrons which can move from one bond to the other in a molecular structure. The structure which is given in the question is an aromatic and aliphatic ring. In the given, we have to discuss delocalised electrons but they should belong to pi electrons.
In case of option A, we can draw the structure of benzene as,
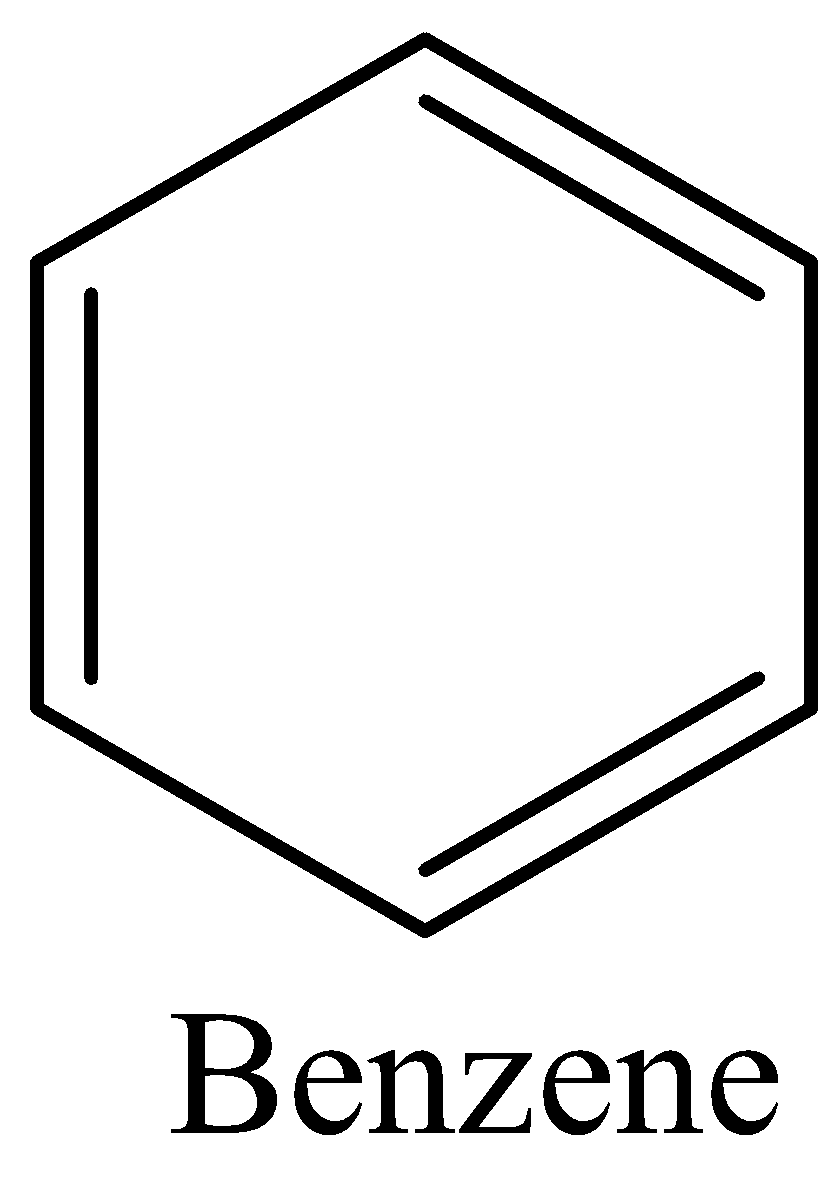
We must remember that the Benzene have equivalent six c-c bonds and 6 electrons which are delocalised around the six carbon atoms and the delocalised electrons are represented by a circle. The aromatic nature of benzene depends on the presence of delocalised $\pi $-electrons. Therefore, the option A is correct.
In case of option B, we can draw the cyclohexane structure as,
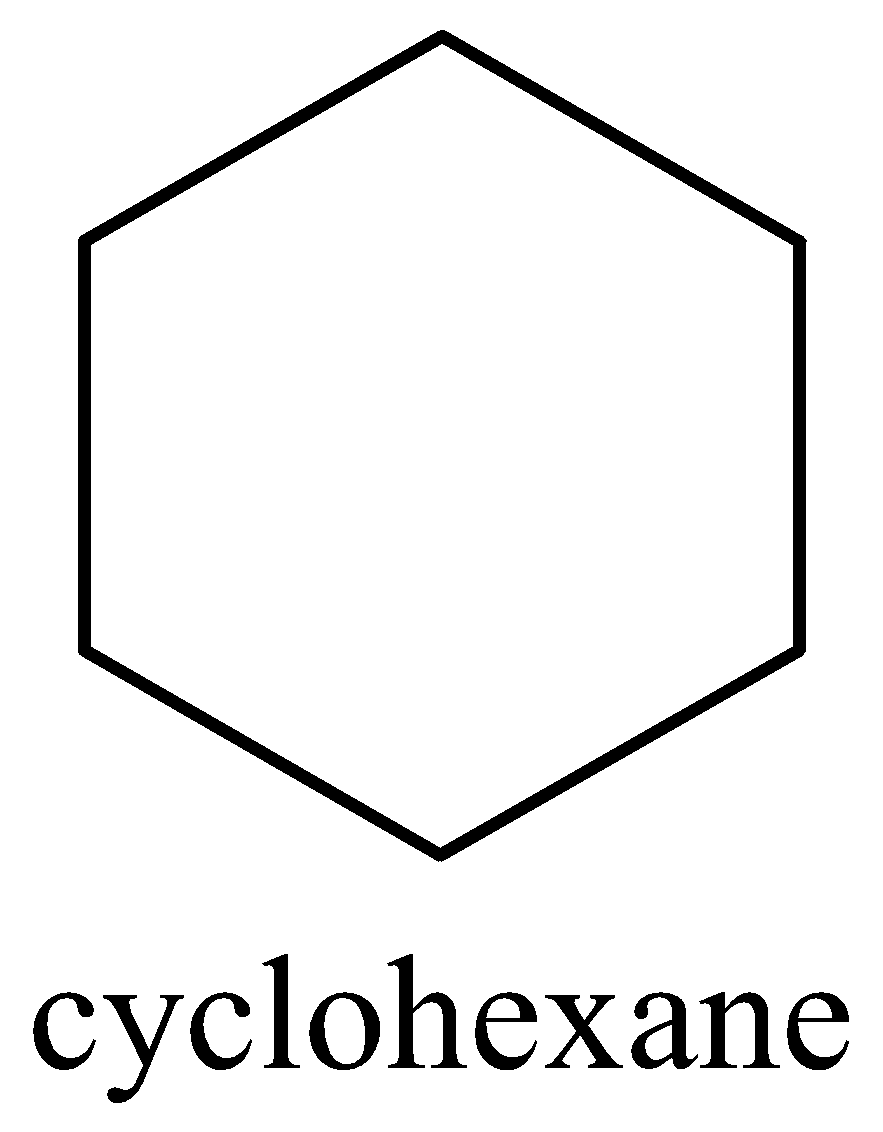
The above structure of cyclohexane, the six C-C bonds are equivalent but it does not have any pi electrons. Therefore, option B is incorrect.
In case of option C, we can draw the structure of methane as,
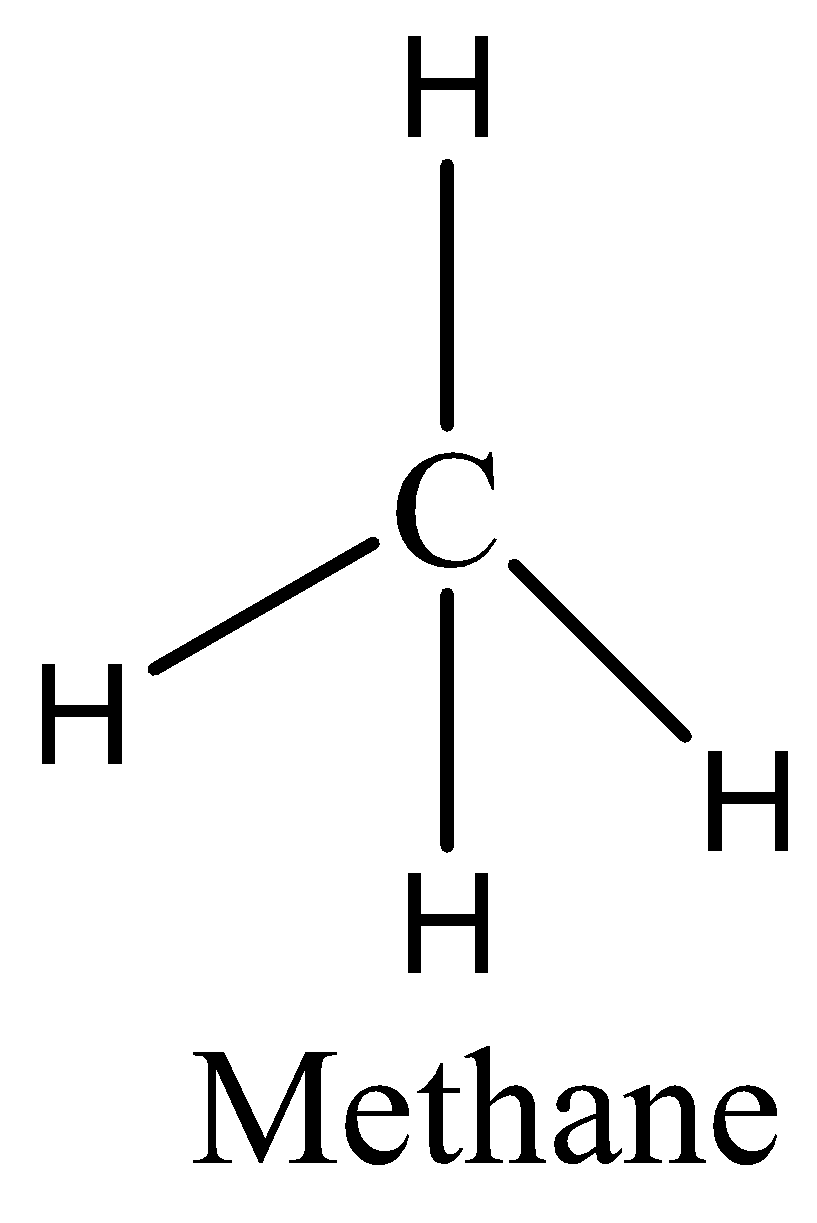
As we know that, the methane has 4 C-H bonds and there are no delocalised electrons. Therefore, the option C is incorrect.
In case of option D, we can draw the structure of ethane as,
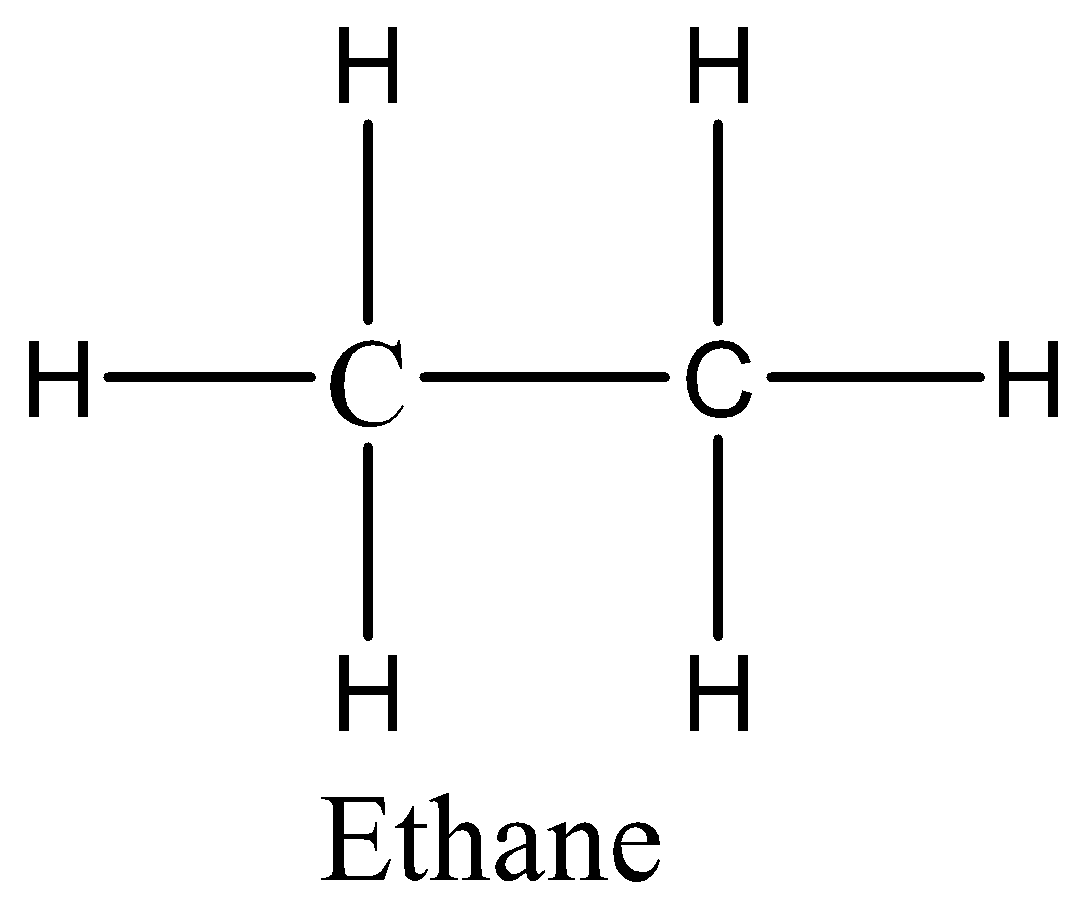
The above structure of ethane clearly shows there is no double bond. Therefore, the option D is incorrect.
Note:
We must remember that the delocalisation of electrons in the molecule leads to resonance structures. These resonance structures play an important role in nucleophilic and electrophilic reactions. We have remembered that the stability of compounds depends on the number of resonating structures present i.e., the number of resonating structures increases the stability of the compound also increases.
Complete answer:
We must remember that the delocalised electrons are those electrons which can move from one bond to the other in a molecular structure. The structure which is given in the question is an aromatic and aliphatic ring. In the given, we have to discuss delocalised electrons but they should belong to pi electrons.
In case of option A, we can draw the structure of benzene as,

We must remember that the Benzene have equivalent six c-c bonds and 6 electrons which are delocalised around the six carbon atoms and the delocalised electrons are represented by a circle. The aromatic nature of benzene depends on the presence of delocalised $\pi $-electrons. Therefore, the option A is correct.
In case of option B, we can draw the cyclohexane structure as,

The above structure of cyclohexane, the six C-C bonds are equivalent but it does not have any pi electrons. Therefore, option B is incorrect.
In case of option C, we can draw the structure of methane as,

As we know that, the methane has 4 C-H bonds and there are no delocalised electrons. Therefore, the option C is incorrect.
In case of option D, we can draw the structure of ethane as,

The above structure of ethane clearly shows there is no double bond. Therefore, the option D is incorrect.
Note:
We must remember that the delocalisation of electrons in the molecule leads to resonance structures. These resonance structures play an important role in nucleophilic and electrophilic reactions. We have remembered that the stability of compounds depends on the number of resonating structures present i.e., the number of resonating structures increases the stability of the compound also increases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




