
Which of the following have +M effect (${e^ - }$ donating mesomeric effect) ?
This question has multiple correct options
(A) $N{O_2}$
(B) $COOH$
(C) $N{H_2}$
(D) $SR$
Answer
590.7k+ views
Hint: The mesomeric effect in chemistry is a property of substituents or functional groups in a chemical compound. It is defined as the polarity produced in the molecule by the interaction of two $Pi(\pi )$ bonds or between a pi bond and lone pair of electrons present on an adjacent atom.
Complete answer:
There are two types of mesomeric effect:
a) $+M$ effect (${e^ - }$ donating effect)
b) $-M$ effect (${e^ - }$ withdrawing effect)
1. $+M$ effect (${e^ - }$ donating effect) – When the electrons or the $\pi $ electrons are transferred from a particular group towards a conjugate system, this increasing the electron density of the conjugate system then such a phenomenon is known as $(+M)$ effect ${e^ - }$ donating mesomeric effect. For the $+M$ effect the group should have a lone pair or negative charge.
Group showing $+M$ effect –
$- NH, - N{H_2} - NHR, - NR, - O, - OH, - OR, - F, - Cl, - SH, - SR$ etc.
2. $-M$ effect (${e^ - }$ donating effect) – When pi-bond electrons are transferred from the conjugate system to a particular group thus the electron density of the conjugate system is discovered, then the phenomenon is known as negative isomeric effect $( - M)$.
Group showing $ - M$ effect –
$- N{O_2}, - CN, - COX, - S{O_3}H, - CHO, - CON{H_2}, - COR, - COOH, - COOR$ etc.
Therefore from the above information
$- N{O_2}$ and $- COOH$ are electron withdrawing groups
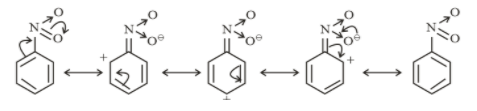
Resonating structures of nitrobenzene
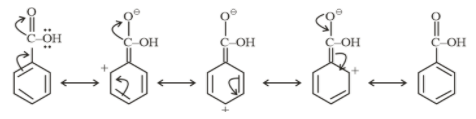
Resonating structure of Benzoic acid
From above structures, it is clear that $- N{O_2}$ and $- COOH$ withdraw electrons from the ring. So they show a $- M$ effect.
$- N{H_2}$ and $ - SR$ are electron donating groups –
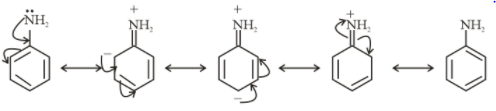
Resonating structures of Aniline
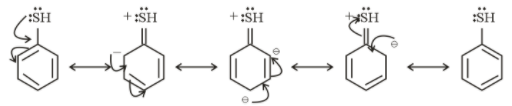
Resonating structure of Thiophenol
From the above structure, it is clear that $ - N{H_2}$ and $ - SH$ donate electrons in the ring. So, they show $ + M$ effect.
Hence, the correct answers are (C) and (D) $( - N{H_2}, - SR)$.
Note: Molecules which have double or triple bond show $ - M$ effect whereas molecules which have lone pair on the directly bonded atom show $ + M$ effect
Complete answer:
There are two types of mesomeric effect:
a) $+M$ effect (${e^ - }$ donating effect)
b) $-M$ effect (${e^ - }$ withdrawing effect)
1. $+M$ effect (${e^ - }$ donating effect) – When the electrons or the $\pi $ electrons are transferred from a particular group towards a conjugate system, this increasing the electron density of the conjugate system then such a phenomenon is known as $(+M)$ effect ${e^ - }$ donating mesomeric effect. For the $+M$ effect the group should have a lone pair or negative charge.
Group showing $+M$ effect –
$- NH, - N{H_2} - NHR, - NR, - O, - OH, - OR, - F, - Cl, - SH, - SR$ etc.
2. $-M$ effect (${e^ - }$ donating effect) – When pi-bond electrons are transferred from the conjugate system to a particular group thus the electron density of the conjugate system is discovered, then the phenomenon is known as negative isomeric effect $( - M)$.
Group showing $ - M$ effect –
$- N{O_2}, - CN, - COX, - S{O_3}H, - CHO, - CON{H_2}, - COR, - COOH, - COOR$ etc.
Therefore from the above information
$- N{O_2}$ and $- COOH$ are electron withdrawing groups

Resonating structures of nitrobenzene

Resonating structure of Benzoic acid
From above structures, it is clear that $- N{O_2}$ and $- COOH$ withdraw electrons from the ring. So they show a $- M$ effect.
$- N{H_2}$ and $ - SR$ are electron donating groups –

Resonating structures of Aniline

Resonating structure of Thiophenol
From the above structure, it is clear that $ - N{H_2}$ and $ - SH$ donate electrons in the ring. So, they show $ + M$ effect.
Hence, the correct answers are (C) and (D) $( - N{H_2}, - SR)$.
Note: Molecules which have double or triple bond show $ - M$ effect whereas molecules which have lone pair on the directly bonded atom show $ + M$ effect
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




