
Which of the following has zero dipole moment ?
A. $B{F_3}$
B. $BeClBr$
C. $C{H_2}C{l_2}$
D. $COS$
Answer
585.9k+ views
Hint: Dipole moment is defined as the product of the magnitude of the charge and the distance between the centre of positive and negative charges. Dipole moment is a measure of the polarity of a molecule .
Complete step by step answer:
Dipole moments arise from electronegativity differences .
it is denoted by $\mu $
$\mu = q \times r$
where , q=magnitude of charge
and r= distance between charges
In the case of polyatomic molecules, $\mu $ not only depends upon the individual dipole moments of the bonds but also on the spatial arrangement of various bonds in the molecule.
When all the bonds in a molecule are oriented in such a way that they totally cancel each other's effects then the dipole moment is said to be zero.
In case of BF3,
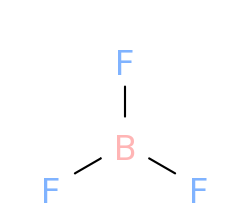
As we can see , the molecule has a trigonal planar shape
That is, the bonds are oriented at an angle of ${120^ \circ }$ such that the resultant dipole due to two $B - F$ bonds cancel the third bond dipole and so the net dipole moment becomes zero.
In all the other molecules given in the options , the bonds are so arranged that the net dipole moment is not equal to zero
So, the correct answer is Option A .
Note:
Dipole moment is used to calculate the polarity of the bonds . The dipole moment of a molecule depends on two things: the individual dipole moment of each bond and the arrangement of the molecules .
Complete step by step answer:
Dipole moments arise from electronegativity differences .
it is denoted by $\mu $
$\mu = q \times r$
where , q=magnitude of charge
and r= distance between charges
In the case of polyatomic molecules, $\mu $ not only depends upon the individual dipole moments of the bonds but also on the spatial arrangement of various bonds in the molecule.
When all the bonds in a molecule are oriented in such a way that they totally cancel each other's effects then the dipole moment is said to be zero.
In case of BF3,

As we can see , the molecule has a trigonal planar shape
That is, the bonds are oriented at an angle of ${120^ \circ }$ such that the resultant dipole due to two $B - F$ bonds cancel the third bond dipole and so the net dipole moment becomes zero.
In all the other molecules given in the options , the bonds are so arranged that the net dipole moment is not equal to zero
So, the correct answer is Option A .
Note:
Dipole moment is used to calculate the polarity of the bonds . The dipole moment of a molecule depends on two things: the individual dipole moment of each bond and the arrangement of the molecules .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




