
Which of the following has intramolecular H-bonding?
A. Ortho-nitrophenol
B. Ortho-boric acid
C. Both (A) and (B)
D. None of these
Answer
533.1k+ views
Hint: Before solving this question, we should first know about Hydrogen-bonding, its types and then we can look at the options and find out which of them has intramolecular H-bonding. The formation of the hydrogen bond is Hydrogen bonding. They are of two types: Intermolecular and Intramolecular.
Complete answer:
Hydrogen- Bonding occurs because of the dipole-dipole interaction between the Hydrogen atom and a strongly electronegative element and another electronegative atom lies in the region of the Hydrogen atom having a lone pair of electrons. Let us now discuss Intramolecular Hydrogen Bonding.
Intramolecular H- Bonding: It is the bonding that occurs between the same molecule. It occurs in compounds which have two groups in which one group has a hydrogen atom joined to an electronegative atom and the other group has a strongly electronegative atom joined to a weak electronegative atom of another group.
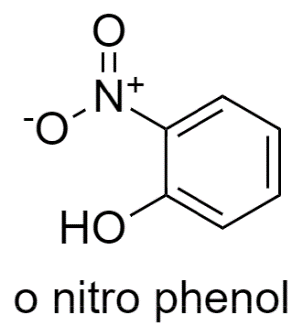
Let us see the first option, In ortho-nitrophenol structure, the donor and the acceptor both are in the same molecule, so Intramolecular Hydrogen bonding is possible in this.
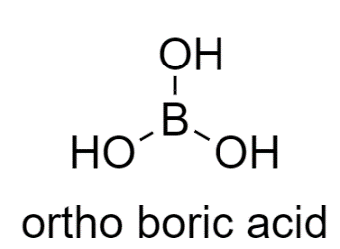
Now moving on to the second option, In ortho-boric acid structure, the donor and the acceptor are not within the same molecule, therefore it shows Intermolecular Hydrogen bonding.
So, the correct answer is “Option A”.
Note:
There is another type of hydrogen bonding i.e. Intermolecular H- Bonding. It is the bonding that occurs between different molecules of the same or different compounds. Some examples of it are Hydrogen bonding in water, alcohol, etc.
Complete answer:
Hydrogen- Bonding occurs because of the dipole-dipole interaction between the Hydrogen atom and a strongly electronegative element and another electronegative atom lies in the region of the Hydrogen atom having a lone pair of electrons. Let us now discuss Intramolecular Hydrogen Bonding.
Intramolecular H- Bonding: It is the bonding that occurs between the same molecule. It occurs in compounds which have two groups in which one group has a hydrogen atom joined to an electronegative atom and the other group has a strongly electronegative atom joined to a weak electronegative atom of another group.
Let us see the first option, In ortho-nitrophenol structure, the donor and the acceptor both are in the same molecule, so Intramolecular Hydrogen bonding is possible in this.

Now moving on to the second option, In ortho-boric acid structure, the donor and the acceptor are not within the same molecule, therefore it shows Intermolecular Hydrogen bonding.

So, the correct answer is “Option A”.
Note:
There is another type of hydrogen bonding i.e. Intermolecular H- Bonding. It is the bonding that occurs between different molecules of the same or different compounds. Some examples of it are Hydrogen bonding in water, alcohol, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




