
Which of the following has a plane of symmetry?
A. $1,4 - $Dimethylcyclohexene
B. $1,3 - $Dimethylcyclohexene
C. Trans$ - 1,3 - $dimethylcyclohexane
D. Cis$ - 1,2 - $dimethylcyclohexane
Answer
471.6k+ views
Hint: In order to solve the question, we need to have knowledge of the structure of organic compounds and IUPAC nomenclature so that we can make the structure of compound mentioned above to check the line of symmetry in them.
Complete step by step answer:
To figure out the answer the question we need to draw the structure of all the compounds option by option.
First, we will draw the compound given in option (A) i.e., $1,4 - $Dimethylcyclohexene:
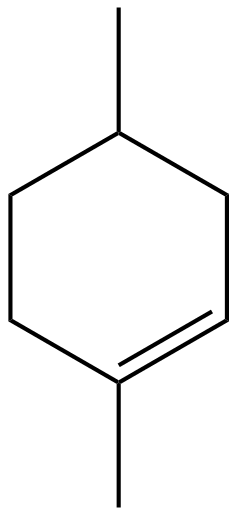
$1,4 - $Dimethylcyclohexene
In this compound, it is clear that there is no line of symmetry. Hence, it is not the desired answer.
Now, we will draw the compound given the option(B) i.e., $1,3 - $Dimethylcyclohexene.
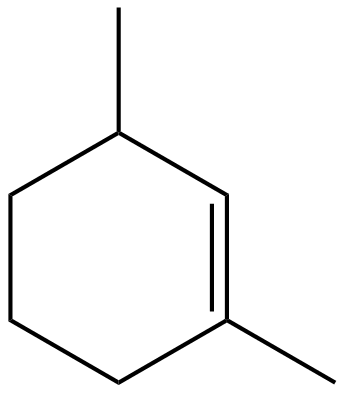
$1,3 - $Dimethylcyclohexene
In this compound after observation, we will find that there is no line of symmetry. Thus, it is not answer we want.
We will draw the compound given the option(C)i.e., Trans$ - 1,3 - $dimethylcyclohexane:
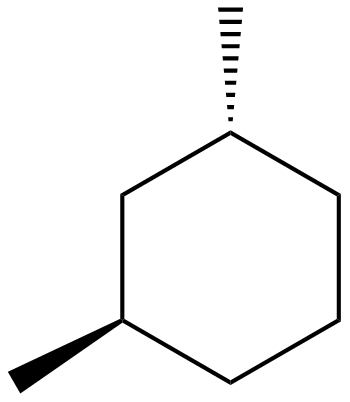
Trans$ - 1,3 - $dimethylcyclohexane
In this option we can see that there is no line of symmetry as this structure is of trans type. So, it is not the answer.
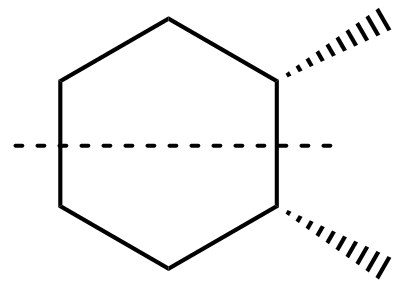
Cis-1,2-dimethylcyclohexane
In this option we can clearly see that a line of symmetry is present in this compound. Therefore, it is the correct answer.
Cis-1,2-dimethylcyclohexane has a plane of symmetry, Hence the option(D) is correct.
Note:
Points to be note while answering these types of questions:
The dashed lines are inside the plane while the thick or black line are on the plane. It is the typical representation for cis and trans compounds.
The compound having a plane of symmetry is optically inactive.
Complete step by step answer:
To figure out the answer the question we need to draw the structure of all the compounds option by option.
First, we will draw the compound given in option (A) i.e., $1,4 - $Dimethylcyclohexene:

$1,4 - $Dimethylcyclohexene
In this compound, it is clear that there is no line of symmetry. Hence, it is not the desired answer.
Now, we will draw the compound given the option(B) i.e., $1,3 - $Dimethylcyclohexene.

$1,3 - $Dimethylcyclohexene
In this compound after observation, we will find that there is no line of symmetry. Thus, it is not answer we want.
We will draw the compound given the option(C)i.e., Trans$ - 1,3 - $dimethylcyclohexane:

Trans$ - 1,3 - $dimethylcyclohexane
In this option we can see that there is no line of symmetry as this structure is of trans type. So, it is not the answer.

Cis-1,2-dimethylcyclohexane
In this option we can clearly see that a line of symmetry is present in this compound. Therefore, it is the correct answer.
Cis-1,2-dimethylcyclohexane has a plane of symmetry, Hence the option(D) is correct.
Note:
Points to be note while answering these types of questions:
The dashed lines are inside the plane while the thick or black line are on the plane. It is the typical representation for cis and trans compounds.
The compound having a plane of symmetry is optically inactive.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




