
Which of the following groups belongs to the actinide series?
(a) Th, Pa, U
(b) Ce, Pr, Nd
(c) Ba, La, Hf
(d) Pt, Au, Ag
Answer
585.6k+ views
Hint: Actinides belongs to the f- block elements and is the second series of f- block elements and involves the filling of (n-2)f subshell and has the general electronic configuration as: $\text{5}{{\text{f}}^{1-14}}\text{6}{{\text{d}}^{0-1}}\text{7}{{\text{s}}^{2}}$.Solve it.
Complete step by step answer:
The elements which involve the filling of f-orbitals i.e. in which the last electron enters the last but two i.e.(n-2) f-subshell of the atom. Their general electronic configuration is:$\text{(n-2)}{{\text{f}}^{1-14}}\text{(n-1)}{{\text{d}}^{0-1}}\text{n}{{\text{s}}^{2}}$. They are also called inner transition elements because they are occurring in between the transition elements.
f-block elements occur in two series: lanthanides and actinides. In lanthanides, the 4f subshell is progressively being filled. It consists of 14 elements from Ce (58) to Lu (71) after La (57) and their general electronic configuration is: $\text{4}{{\text{f}}^{1-14}}\text{5}{{\text{d}}^{0-1}}\text{6}{{\text{s}}^{2}}$. On the other hand, in cases of actinides, the 5f subshell is progressively being filled. It also consists of 14 elements from Th (90) to Lr (103) after Ac (89) and their general electronic configuration is: $\text{5}{{\text{f}}^{1-14}}\text{6}{{\text{d}}^{0-1}}\text{7}{{\text{s}}^{2}}$
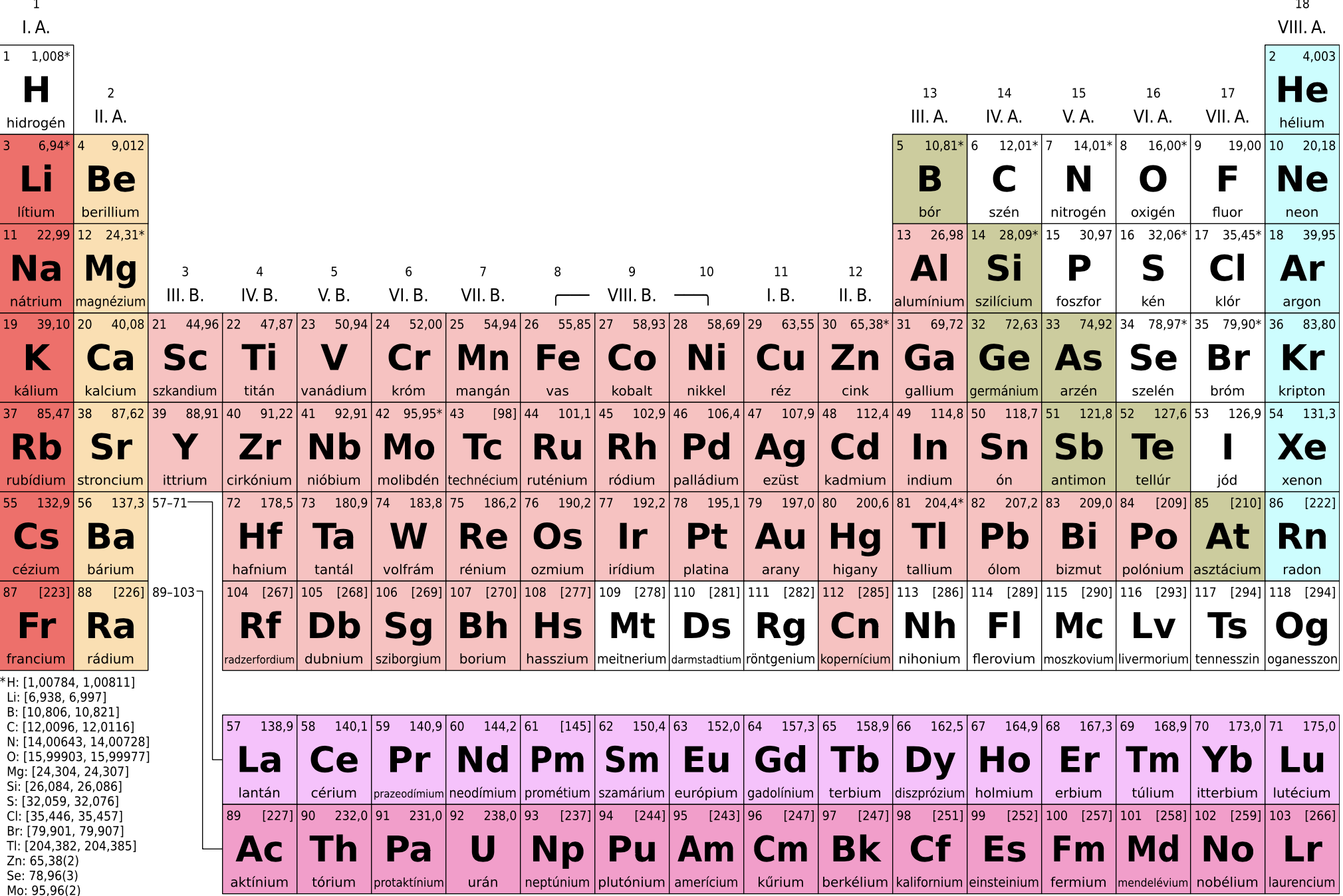
From the above period table, we can see that the thorium (Th), protactinium (Pa), and uranium(U) group belongs to the actinide series.
Hence, option (a) is correct.
Note: In actinides, there is steady decrease in the ionic radii with the increase in the atomic number and this is known as actinide contraction i.e. the nuclear charge increases as we move from left to right but the electrons are also added in the n-2 subshell and the effect of increased nuclear charge dominates over the imperfect shielding by the 5f electrons and thus, the 5f electron subshell contracts and thus, the decrease is very small
Complete step by step answer:
The elements which involve the filling of f-orbitals i.e. in which the last electron enters the last but two i.e.(n-2) f-subshell of the atom. Their general electronic configuration is:$\text{(n-2)}{{\text{f}}^{1-14}}\text{(n-1)}{{\text{d}}^{0-1}}\text{n}{{\text{s}}^{2}}$. They are also called inner transition elements because they are occurring in between the transition elements.
f-block elements occur in two series: lanthanides and actinides. In lanthanides, the 4f subshell is progressively being filled. It consists of 14 elements from Ce (58) to Lu (71) after La (57) and their general electronic configuration is: $\text{4}{{\text{f}}^{1-14}}\text{5}{{\text{d}}^{0-1}}\text{6}{{\text{s}}^{2}}$. On the other hand, in cases of actinides, the 5f subshell is progressively being filled. It also consists of 14 elements from Th (90) to Lr (103) after Ac (89) and their general electronic configuration is: $\text{5}{{\text{f}}^{1-14}}\text{6}{{\text{d}}^{0-1}}\text{7}{{\text{s}}^{2}}$

From the above period table, we can see that the thorium (Th), protactinium (Pa), and uranium(U) group belongs to the actinide series.
Hence, option (a) is correct.
Note: In actinides, there is steady decrease in the ionic radii with the increase in the atomic number and this is known as actinide contraction i.e. the nuclear charge increases as we move from left to right but the electrons are also added in the n-2 subshell and the effect of increased nuclear charge dominates over the imperfect shielding by the 5f electrons and thus, the 5f electron subshell contracts and thus, the decrease is very small
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




