
Which of the following gases is lighter than air?
A. Oxygen
B. Hydrogen chloride
C. Ammonia
D. Chlorine
Answer
611.7k+ views
Hint: Here, we will first of all, we will see the definition of atomic mass. Then calculate the atomic mass of given compounds . Assuming that air consists mainly of nitrogen(78%). The Gases which have less atomic mass than that of air will be lighter than the air.
Complete answer:
First of all, we will see the definition of atomic mass.
Atomic mass, the quantity of matter contained in an atom of an element. It is expressed as a multiple of one-twelfth the mass of the carbon-12 atom, $1.992646547 \times {10^{−23}}$ gram, which is assigned an atomic mass of 12 units. In this scale 1 atomic mass unit (amu) corresponds to $1.660539040 \times {10^{−24}}$ gram.
For example, the atomic mass of carbon(C) is 12 units.
Let us consider us consider that air mainly consists of nitrogen gas (${N_2}$)
Atomic mass of nitrogen gas (${N_2}$) = $2 \times 14$ =28
Atomic mass of hydrogen chloride(HCl) = 1+35.5=36.5
Atomic mass of Ammonia ($N{H_3}$ ) = 14+3$ \times $1=17
Atomic mass of Chlorine gas (Cl) = 35.5
Therefore, we can say that only Ammonia out of four given options has less mass than that of air.
So. Option C is the correct one.
Note: You should remember the atomic mass of some important elements.
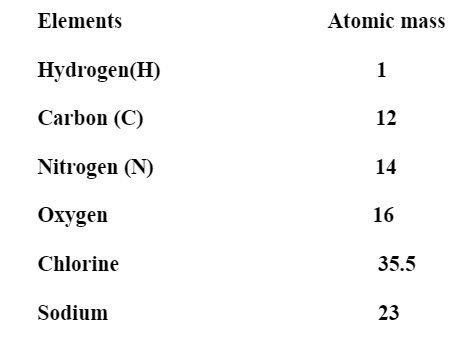
On going down the group, the atomic mass increases due to an increase in the number of protons and neutrons.
Complete answer:
First of all, we will see the definition of atomic mass.
Atomic mass, the quantity of matter contained in an atom of an element. It is expressed as a multiple of one-twelfth the mass of the carbon-12 atom, $1.992646547 \times {10^{−23}}$ gram, which is assigned an atomic mass of 12 units. In this scale 1 atomic mass unit (amu) corresponds to $1.660539040 \times {10^{−24}}$ gram.
For example, the atomic mass of carbon(C) is 12 units.
Let us consider us consider that air mainly consists of nitrogen gas (${N_2}$)
Atomic mass of nitrogen gas (${N_2}$) = $2 \times 14$ =28
Atomic mass of hydrogen chloride(HCl) = 1+35.5=36.5
Atomic mass of Ammonia ($N{H_3}$ ) = 14+3$ \times $1=17
Atomic mass of Chlorine gas (Cl) = 35.5
Therefore, we can say that only Ammonia out of four given options has less mass than that of air.
So. Option C is the correct one.
Note: You should remember the atomic mass of some important elements.

On going down the group, the atomic mass increases due to an increase in the number of protons and neutrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




