
Which of the following Fischer’s projection formula is identical to D-glyceraldehyde?
(a)
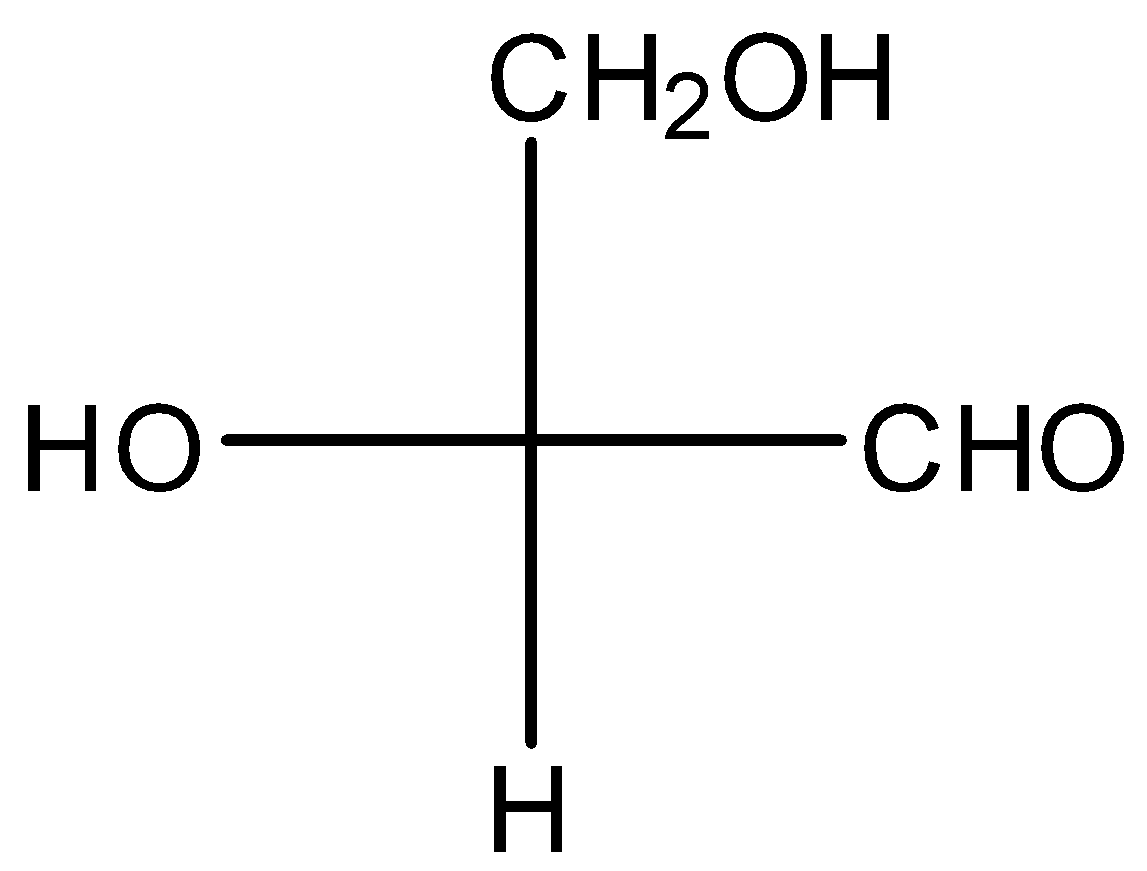
(b)
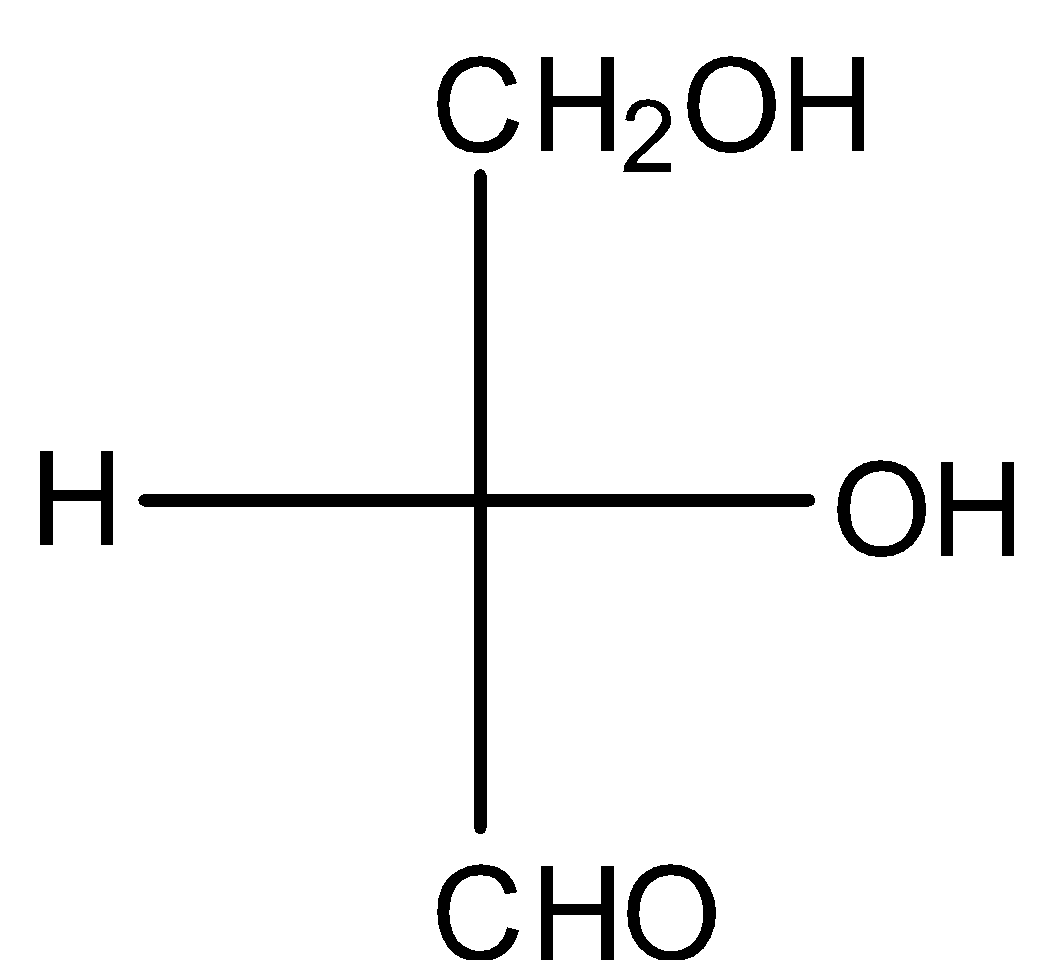
(C)
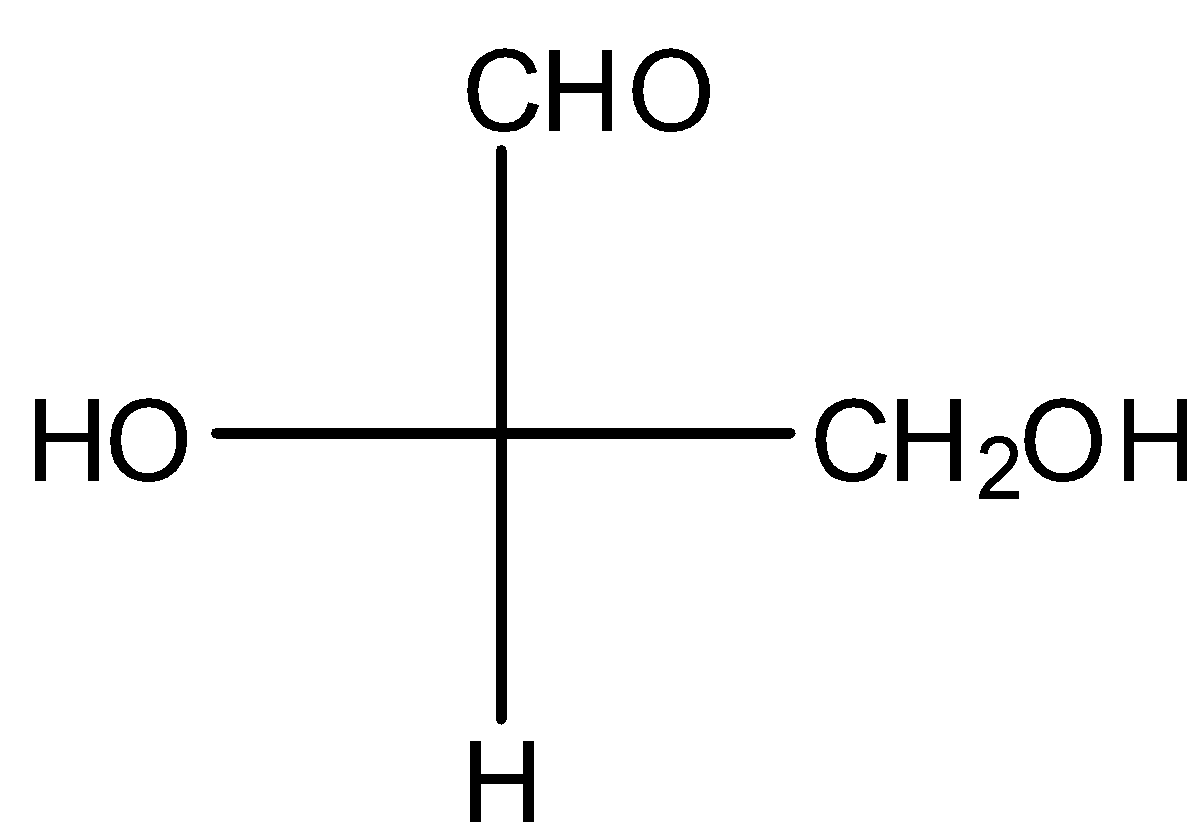
(D)
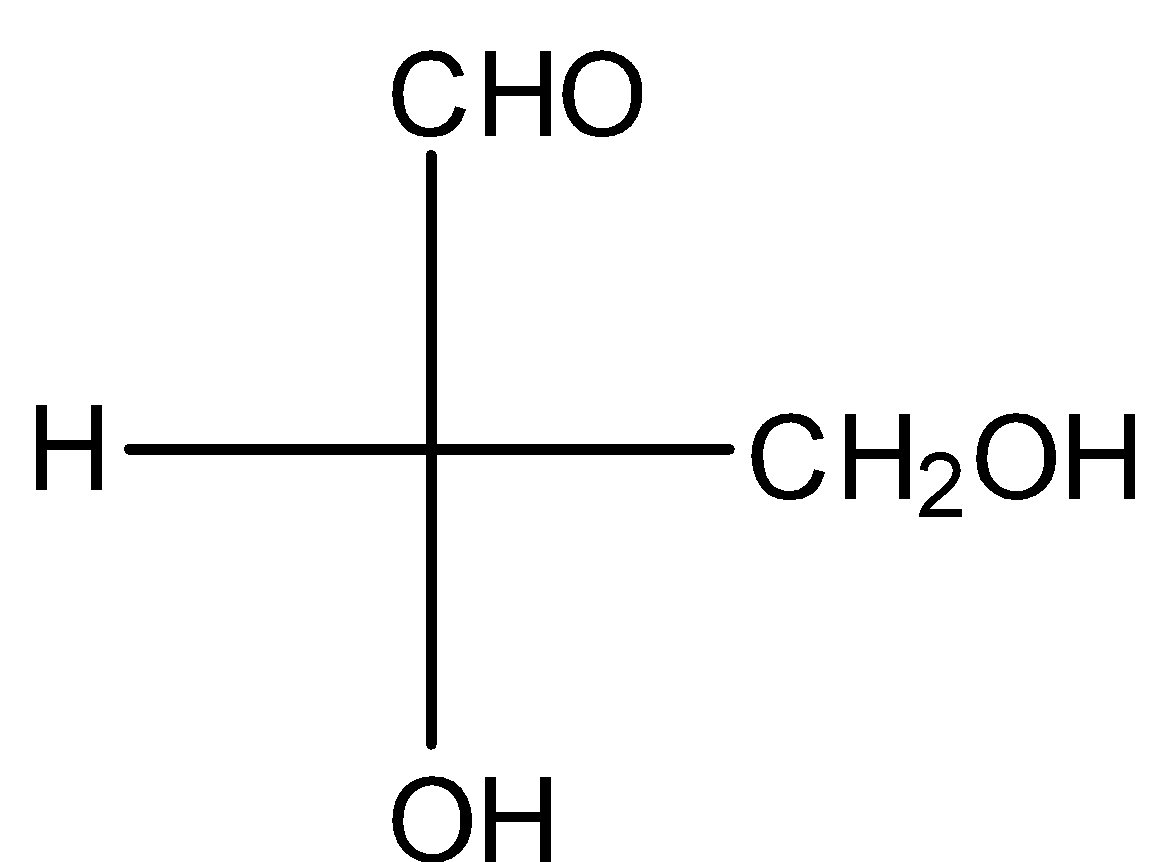




Answer
570k+ views
Hint: We know that there are two types of glyceraldehydes namely L-glyceraldehyde and D-glyceraldehyde. Both are enantiomers. In L-Glyceraldehyde, OH group is present at the left and H group is at the right but in D-glyceraldehyde OH is present at the right and H is at the left.
Complete step by step answer:
Let’s understand the difference of L-glyceraldehyde and D-glyceraldehyde with the help of diagrams.

From the above structure, we see that in both the enantiomers CHO group is on the upper side and $CH_2OH$ is on the lower side. But, the position of OH and H is different in both the enantiomers.
Now, come to the question. Here, we have to identify the Fisher projection which is identical to D-glyceraldehyde.
We know that, if even number of interchanges is done in the Fisher projection formula we get the identical molecule.
For the Fischer projection in option (C),

Here, CHO group is in the vertical position. So, we don’t need to exchange it. Now, we have to interchange H and $CH_2OH$. Then, L-glyceraldehyde forms. Now, if we interchange the position of H and OH D-glyceraldehyde obtained. So, 2 times interchanges give the D-glyceraldehyde.
All other Fisher projections result in D-glyceraldehyde in an odd number of interchanges.
So, the correct answer is Option c .
Note: Always remember that Fischer projection represents three dimensional carbon atoms in 2-D. The molecule is drawn the form of a cross. The tetrahedral carbon is in the plane of the paper at the centre of the cross. Atoms bonded horizontally to the tetrahedral carbon are behind the plane of paper and vertically bonded atoms are in front of the paper.
Complete step by step answer:
Let’s understand the difference of L-glyceraldehyde and D-glyceraldehyde with the help of diagrams.

From the above structure, we see that in both the enantiomers CHO group is on the upper side and $CH_2OH$ is on the lower side. But, the position of OH and H is different in both the enantiomers.
Now, come to the question. Here, we have to identify the Fisher projection which is identical to D-glyceraldehyde.
We know that, if even number of interchanges is done in the Fisher projection formula we get the identical molecule.
For the Fischer projection in option (C),

Here, CHO group is in the vertical position. So, we don’t need to exchange it. Now, we have to interchange H and $CH_2OH$. Then, L-glyceraldehyde forms. Now, if we interchange the position of H and OH D-glyceraldehyde obtained. So, 2 times interchanges give the D-glyceraldehyde.
All other Fisher projections result in D-glyceraldehyde in an odd number of interchanges.
So, the correct answer is Option c .
Note: Always remember that Fischer projection represents three dimensional carbon atoms in 2-D. The molecule is drawn the form of a cross. The tetrahedral carbon is in the plane of the paper at the centre of the cross. Atoms bonded horizontally to the tetrahedral carbon are behind the plane of paper and vertically bonded atoms are in front of the paper.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




