
Which of the following events does not occur during \[S{{N}^{2}}\]reaction mechanism?
a.) Backside attack of nucleophile
b.) Formation of carbonium ion
c.) One step continuous process
d.) 100% inversion of configuration
Answer
585k+ views
Hint: Here \[S{{N}^{2}}\] is nucleophilic substitution bimolecular reaction where a bond is broken and another is formed synchronously. Here we have to talk about the different steps involved in this mechanism.
Complete step by step solution:
In Simple terms in this mechanism, one bond is broken and one bond is formed synchronously, so it is called a nucleophilic substitution bimolecular reaction.
In this reaction there is a backside attack by the nucleophile on the substrate. The nucleophile approaches the given substrate at an angle of \[{{180}^{o}}\] to the carbon-leaving group bond. The carbon - nucleophile bond forms and carbon-leaving group bond breaks simultaneously through a transition state.
Then the leaving group is pushed out of the transition state on the opposite side of the carbon-nucleophile bond, forming the required product. It is important to note that the product is formed with an inversion of the tetrahedral geometry at the atom in the centre.
Let’s take an example of nucleophilic substitution of chloroethane with bromine acting as the nucleophile is:
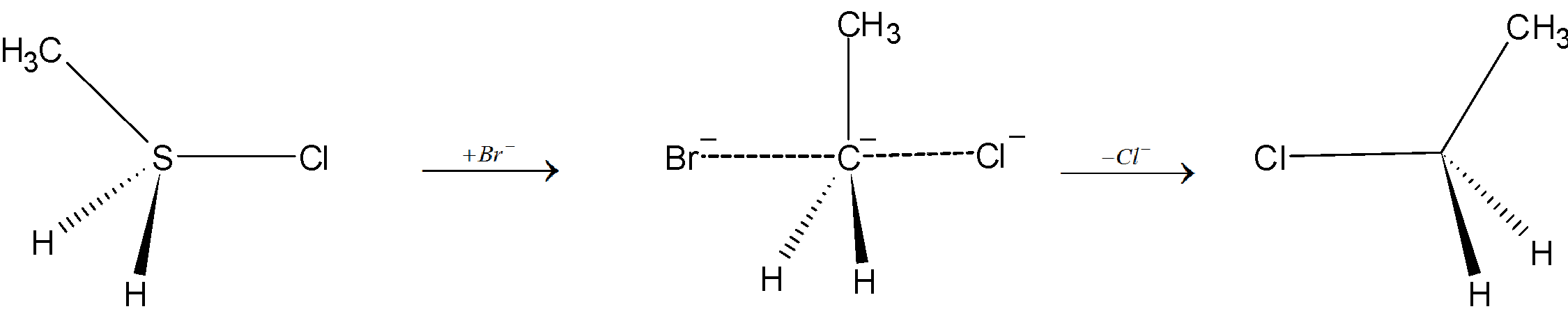
So, from the above explanation we can say that option “B” is the correct answer.
Note: Two reacting species are involved in the slow (rate-determining) step, this leads to the term substitution nucleophilic (bi-molecular) or\[S{{N}^{2}}\]. There is one another major nucleophilic substitution reaction\[S{{N}^{1}}\].
Complete step by step solution:
In Simple terms in this mechanism, one bond is broken and one bond is formed synchronously, so it is called a nucleophilic substitution bimolecular reaction.
In this reaction there is a backside attack by the nucleophile on the substrate. The nucleophile approaches the given substrate at an angle of \[{{180}^{o}}\] to the carbon-leaving group bond. The carbon - nucleophile bond forms and carbon-leaving group bond breaks simultaneously through a transition state.
Then the leaving group is pushed out of the transition state on the opposite side of the carbon-nucleophile bond, forming the required product. It is important to note that the product is formed with an inversion of the tetrahedral geometry at the atom in the centre.
Let’s take an example of nucleophilic substitution of chloroethane with bromine acting as the nucleophile is:

So, from the above explanation we can say that option “B” is the correct answer.
Note: Two reacting species are involved in the slow (rate-determining) step, this leads to the term substitution nucleophilic (bi-molecular) or\[S{{N}^{2}}\]. There is one another major nucleophilic substitution reaction\[S{{N}^{1}}\].
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




