
Which of the following ethers is the most unreactive to cleavage with conc. HBr?
A.
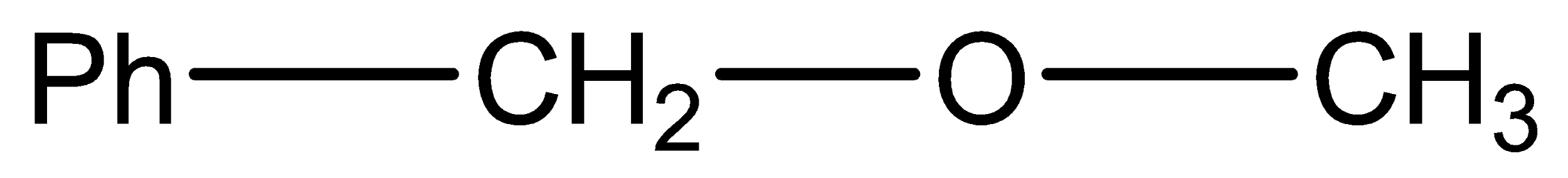
B.
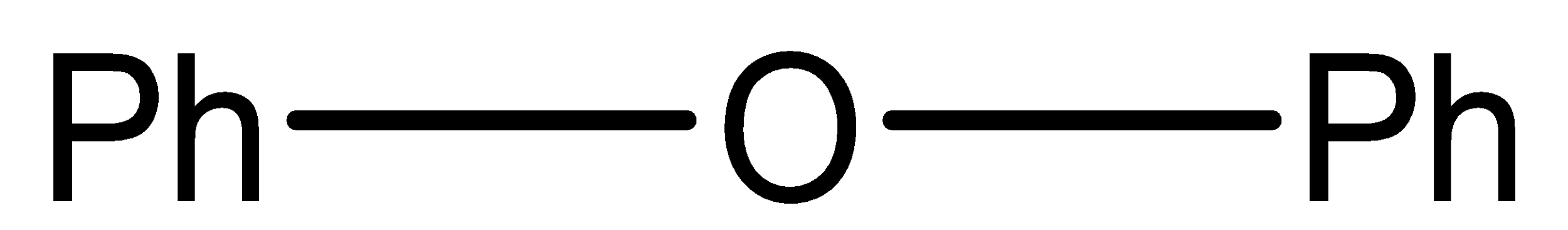
C.
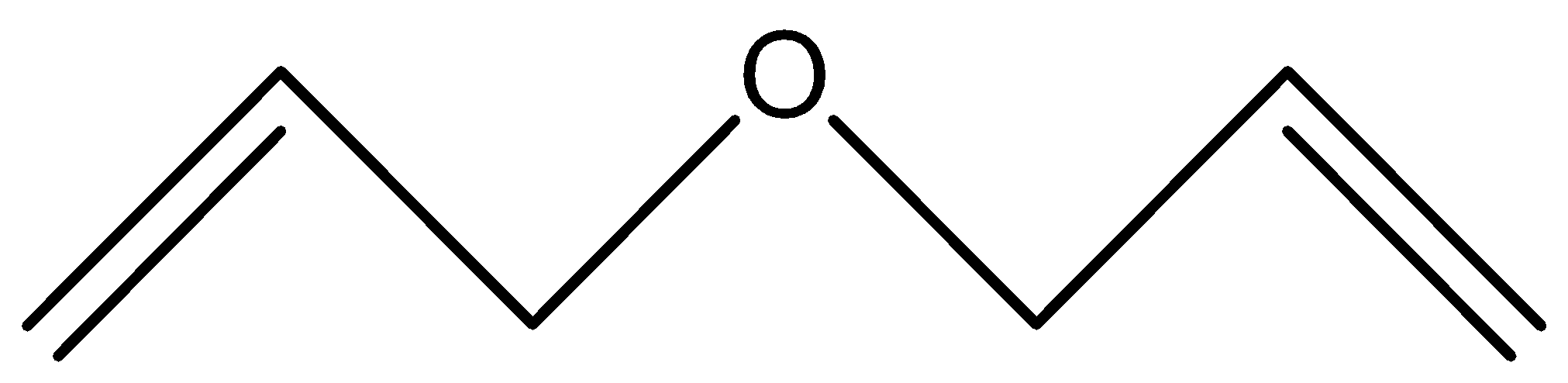
D.
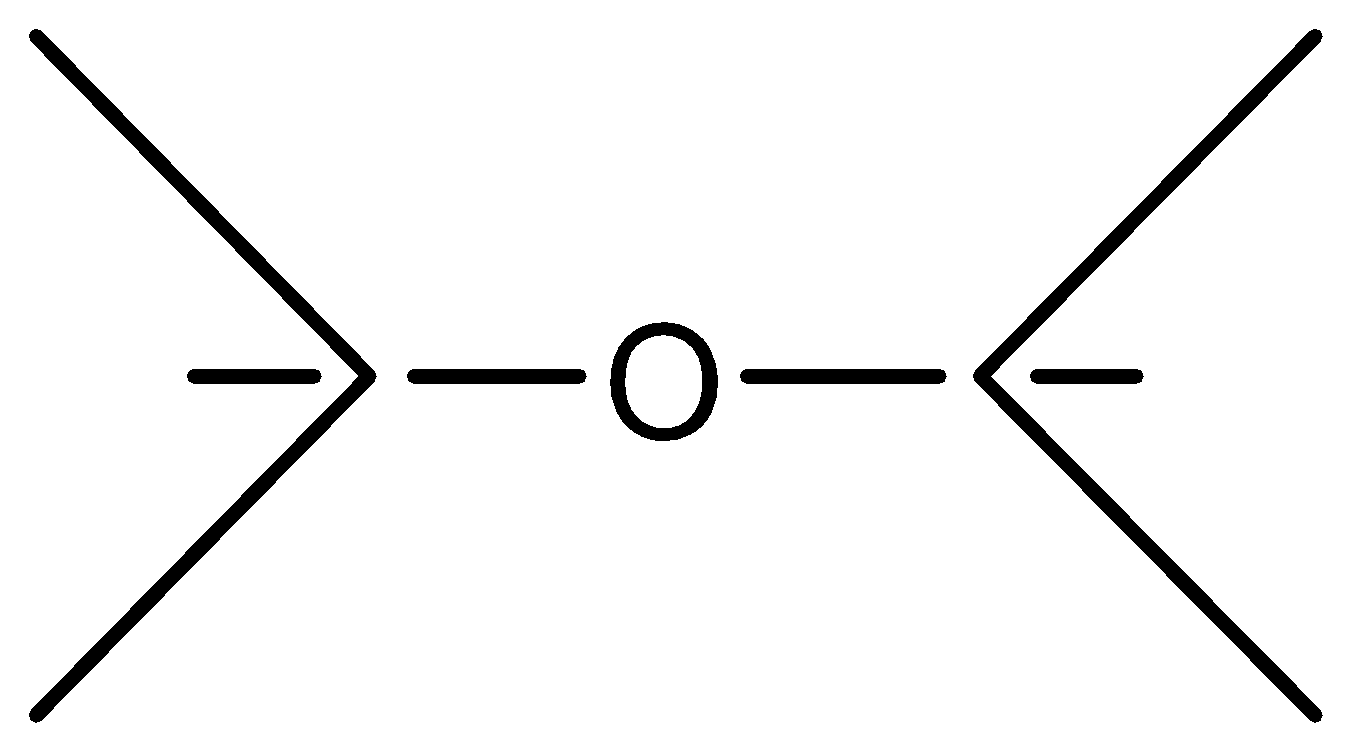
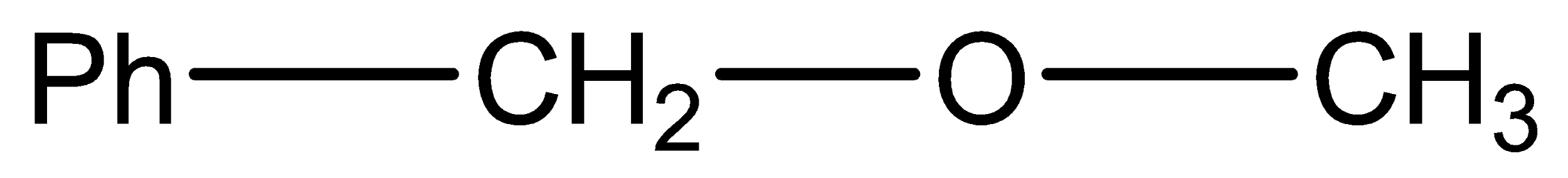
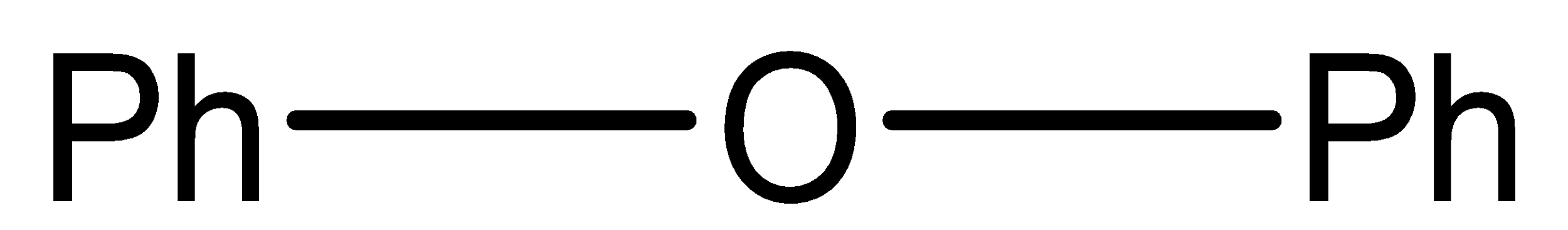


Answer
576.6k+ views
Hint: Diphenyl ether cannot be cleaved by concentrated HBr, it is because in diphenyl ether Oxygen (O) is attached to both phenyl ring and thus the nucleophilic attack is not feasible and also positive charge on benzene unstable.
Complete step by step answer:
Diphenyl ether is the most stable one, and to break bond with phenyl required larger energy.
Alkyl ethers are cleaved by the strong acids that are HI (Hydrogen iodide) or HBr (Hydrogen Bromide) in nucleophilic substitution reactions similar to alcohols. Protonation of the ethereal oxygen creates a good leaving group, a neutral alcohol molecule. The halide ion, bromide or iodide are both good nucleophiles. Considering the structure of the alkyl groups, the reaction can be SN1 or SN2 like. Ethers are highly stable and cannot be claimed or dissociated by HBr or HI, So, it needs concentrated HBr or with dilute \[{H_2}S{O_4}\] phenyl attached with ether has an invisible double bond with O and it forms a very unstable product if O is removed and so as we know every compounds need to be stable so diphenyl ether least reactive to HBr because of its Phenyl.
So, the correct answer is option (B).
Note: In an acid/base reaction, protonation of the alcoholic oxygen to make a better leaving group. This step is very fast and reversible. The lone pairs on the oxygen make it a Lewis base. The bromide ion functions as the nucleophile and attacks to displace the good leaving group, neutral alcohol molecule, by cleaving the \[C - O\] bond. This results in the formation of an alkyl bromide and an alcohol.
Complete step by step answer:
Diphenyl ether is the most stable one, and to break bond with phenyl required larger energy.
Alkyl ethers are cleaved by the strong acids that are HI (Hydrogen iodide) or HBr (Hydrogen Bromide) in nucleophilic substitution reactions similar to alcohols. Protonation of the ethereal oxygen creates a good leaving group, a neutral alcohol molecule. The halide ion, bromide or iodide are both good nucleophiles. Considering the structure of the alkyl groups, the reaction can be SN1 or SN2 like. Ethers are highly stable and cannot be claimed or dissociated by HBr or HI, So, it needs concentrated HBr or with dilute \[{H_2}S{O_4}\] phenyl attached with ether has an invisible double bond with O and it forms a very unstable product if O is removed and so as we know every compounds need to be stable so diphenyl ether least reactive to HBr because of its Phenyl.
So, the correct answer is option (B).
Note: In an acid/base reaction, protonation of the alcoholic oxygen to make a better leaving group. This step is very fast and reversible. The lone pairs on the oxygen make it a Lewis base. The bromide ion functions as the nucleophile and attacks to displace the good leaving group, neutral alcohol molecule, by cleaving the \[C - O\] bond. This results in the formation of an alkyl bromide and an alcohol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




