
Which of the following electron configurations has maximum energy ?
A.
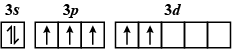
B.
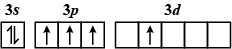
C.
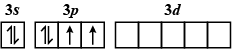
D.
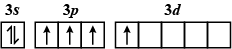




Answer
572.1k+ views
Hint: Energy of orbitals and shell: 1s is the least energy orbital and it is close to the nucleus. 1s is first then second shell, which consists of $2s$ and $2p$. The second shell has high energy and is away from the nucleus than the first shell.
Complete step by step solution:
The order of increasing energy of the sub-atomic orbitals is \[s < p < d < f\].
The energy in an excited state is more than that in the ground state.
So, the correct answer is A.
Additional information:
Each orbital in an atom is considered by a unique set of values of the three quantum numbers $n,l$ and $m$, which resemble the electron's energy, angular momentum, and angular momentum vector components . Each orbital can be engaged by a maximum of two electrons, each with its own spin quantum number. The simple names $s$ orbital, $p$ orbital, $d$ orbital, and $f$ orbital refer to orbitals with angular momentum quantum numbers having $l = 0,1,2,3$ respectively. The names together along the value of n are used to describe the electron configurations of atoms. These are derived from the description by early spectroscopists of certain series of alkali metal .Orbitals for $l > 3$ continue alphabetically.
Note: Atomic orbitals are known as the basic building blocks of the atomic orbital model which is also known as the electron cloud or wave mechanics model. It is a modern framework for visualizing the smallest behavior of electrons. In this model the electron cloud of a multi-electron atom is seen as being built up in an electron configuration which is a product of simpler hydrogen-like atomic orbitals.
Complete step by step solution:
The order of increasing energy of the sub-atomic orbitals is \[s < p < d < f\].
The energy in an excited state is more than that in the ground state.
So, the correct answer is A.
Additional information:
Each orbital in an atom is considered by a unique set of values of the three quantum numbers $n,l$ and $m$, which resemble the electron's energy, angular momentum, and angular momentum vector components . Each orbital can be engaged by a maximum of two electrons, each with its own spin quantum number. The simple names $s$ orbital, $p$ orbital, $d$ orbital, and $f$ orbital refer to orbitals with angular momentum quantum numbers having $l = 0,1,2,3$ respectively. The names together along the value of n are used to describe the electron configurations of atoms. These are derived from the description by early spectroscopists of certain series of alkali metal .Orbitals for $l > 3$ continue alphabetically.
Note: Atomic orbitals are known as the basic building blocks of the atomic orbital model which is also known as the electron cloud or wave mechanics model. It is a modern framework for visualizing the smallest behavior of electrons. In this model the electron cloud of a multi-electron atom is seen as being built up in an electron configuration which is a product of simpler hydrogen-like atomic orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




