
Which of the following does not show geometrical isomerism?
(A)- ${\text{1,2 - Dichloropent - 1 - ene}}$
(B)- ${\text{1,3 - Dichloropent - 2 - ene}}$
(C)- ${\text{1,1 - Dichloropent - 1 - ene}}$
(D)- ${\text{1,4 - Dichloropent - 2 - ene}}$
Answer
570k+ views
Hint: Those compounds in which same groups are attached to the one carbon atom of the two double bonded carbon atoms doesn’t show geometrical isomerism or cis – trans isomerism.
Complete answer:
Geometrical isomerism is a kind of configuration isomerism in which different configuration of groups takes place & in other term it is also known as cis or trans isomerism.
-In option (A) ${\text{1,2 - Dichloropent - 1 - ene}}$ compound is given and it shows geometrical isomerism because they have different groups attached to the double bonded carbon atoms. Structure of cis & trans isomer of this compound is displayed as follow:
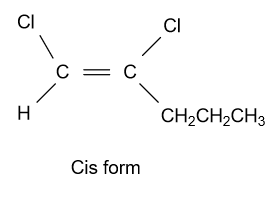
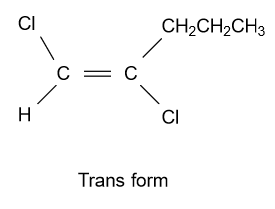
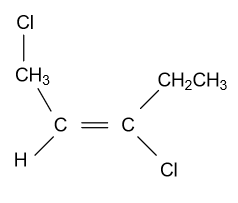
-In option (B) ${\text{1,3 - Dichloropent - 2 - ene}}$ compound is given and it shows geometrical isomerism because they have all four different groups attached to the double bonded carbon atoms. Structure of this compound is displayed as follow:
-In option (C) ${\text{1,1 - Dichloropent - 1 - ene}}$compound is given and it doesn’t show geometrical isomerism because they have the same groups attached to the one of the double bonded carbon atoms. So, it is not able to show cis & trans isomer.
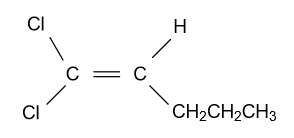
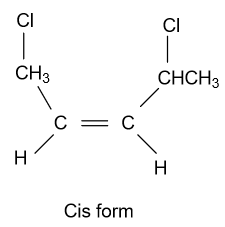
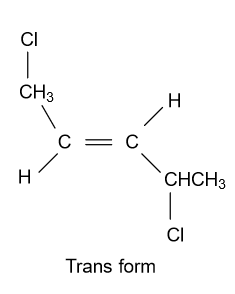
-In option (D) ${\text{1,4 - Dichloropent - 2 - ene}}$compound is given and it shows geometrical isomerism because they have different groups attached to the double bonded carbon atoms. Structure of cis & trans isomer of this compound is displayed as follow:
Note:
Here in the option (B) some of you may think that this will not show geometrical isomerism because they have all four groups are different, but this is not true because these groups also show cis & trans form on the basis of provided sequence to the attached groups.
Complete answer:
Geometrical isomerism is a kind of configuration isomerism in which different configuration of groups takes place & in other term it is also known as cis or trans isomerism.
-In option (A) ${\text{1,2 - Dichloropent - 1 - ene}}$ compound is given and it shows geometrical isomerism because they have different groups attached to the double bonded carbon atoms. Structure of cis & trans isomer of this compound is displayed as follow:


-In option (B) ${\text{1,3 - Dichloropent - 2 - ene}}$ compound is given and it shows geometrical isomerism because they have all four different groups attached to the double bonded carbon atoms. Structure of this compound is displayed as follow:

-In option (C) ${\text{1,1 - Dichloropent - 1 - ene}}$compound is given and it doesn’t show geometrical isomerism because they have the same groups attached to the one of the double bonded carbon atoms. So, it is not able to show cis & trans isomer.

-In option (D) ${\text{1,4 - Dichloropent - 2 - ene}}$compound is given and it shows geometrical isomerism because they have different groups attached to the double bonded carbon atoms. Structure of cis & trans isomer of this compound is displayed as follow:


Note:
Here in the option (B) some of you may think that this will not show geometrical isomerism because they have all four groups are different, but this is not true because these groups also show cis & trans form on the basis of provided sequence to the attached groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




