
Which of the following does not react with \[{\text{HN}}{{\text{O}}_{\text{2}}}\]?
A)
B)
C)
D)




Answer
510k+ views
Hint: Arrhenius is one of the concepts for acid and bases. Swedish chemist Arrhenius is the world's first chemist to talk about acid and bases. Depending on his concept and limitations later so many concepts are developed. Even Arrhenius is the birthplace of the concept of acids and bases. Followed by Arrhenius chemist the concept of acids and bases discuss Bronsted and Lowry in the world.
Complete answer:
According to the Arrhenius concept, one substance said to be acidic means acid is nothing but a substance that dissociates to give hydrogen ions when decomposed in the water.
Examples of Arrhenius acids are hydrochloric acid (\[{\text{HCl}}\]), sulphuric acid (\[{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\]), nitrous acid (\[{\text{HN}}{{\text{O}}_2}\]) etc,.
Hence, \[{\text{HN}}{{\text{O}}_2}\] is acidic.
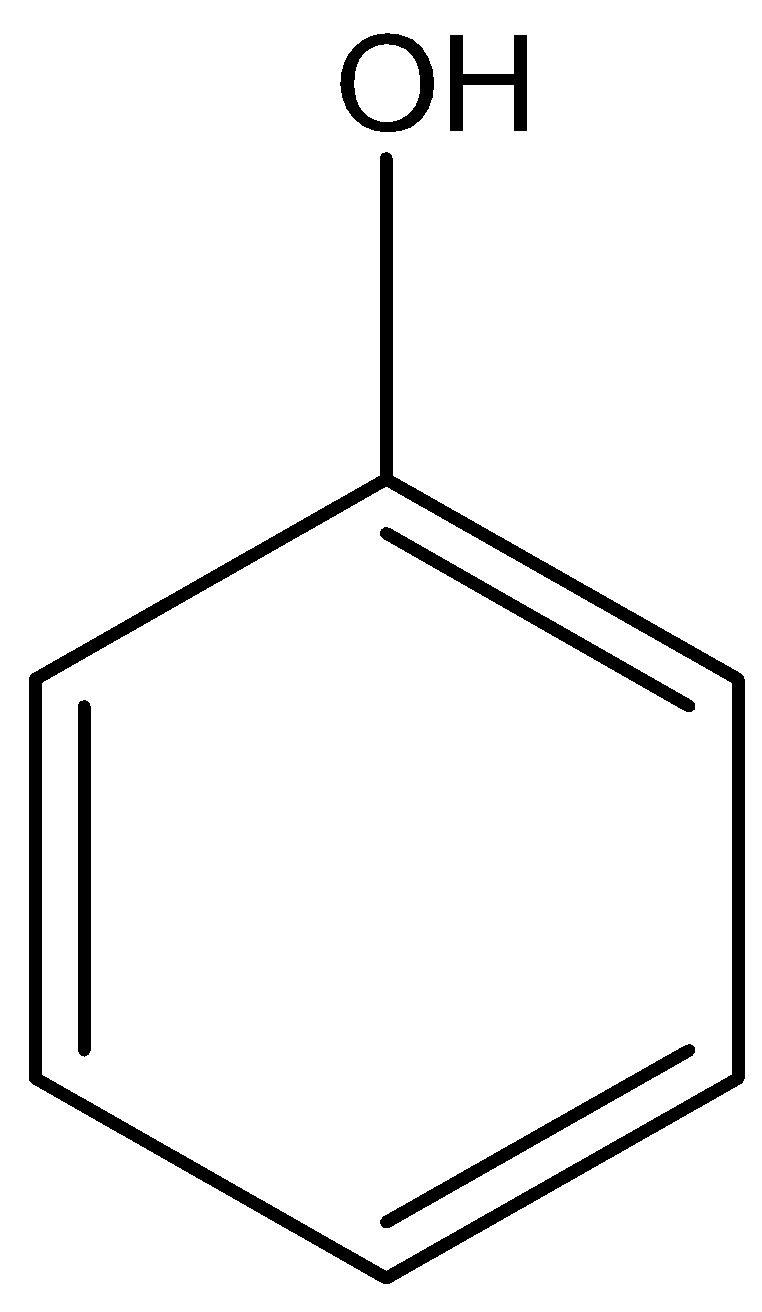
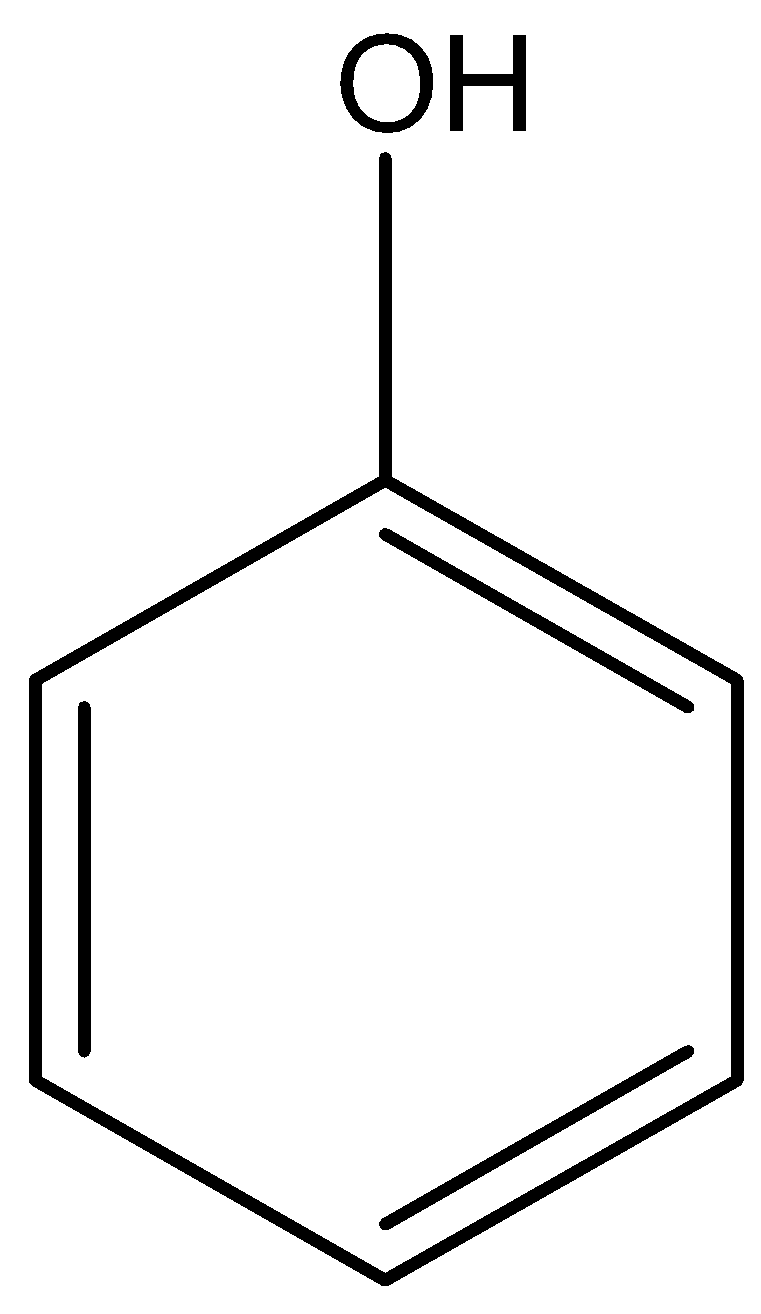
The molecular formula of phenol is
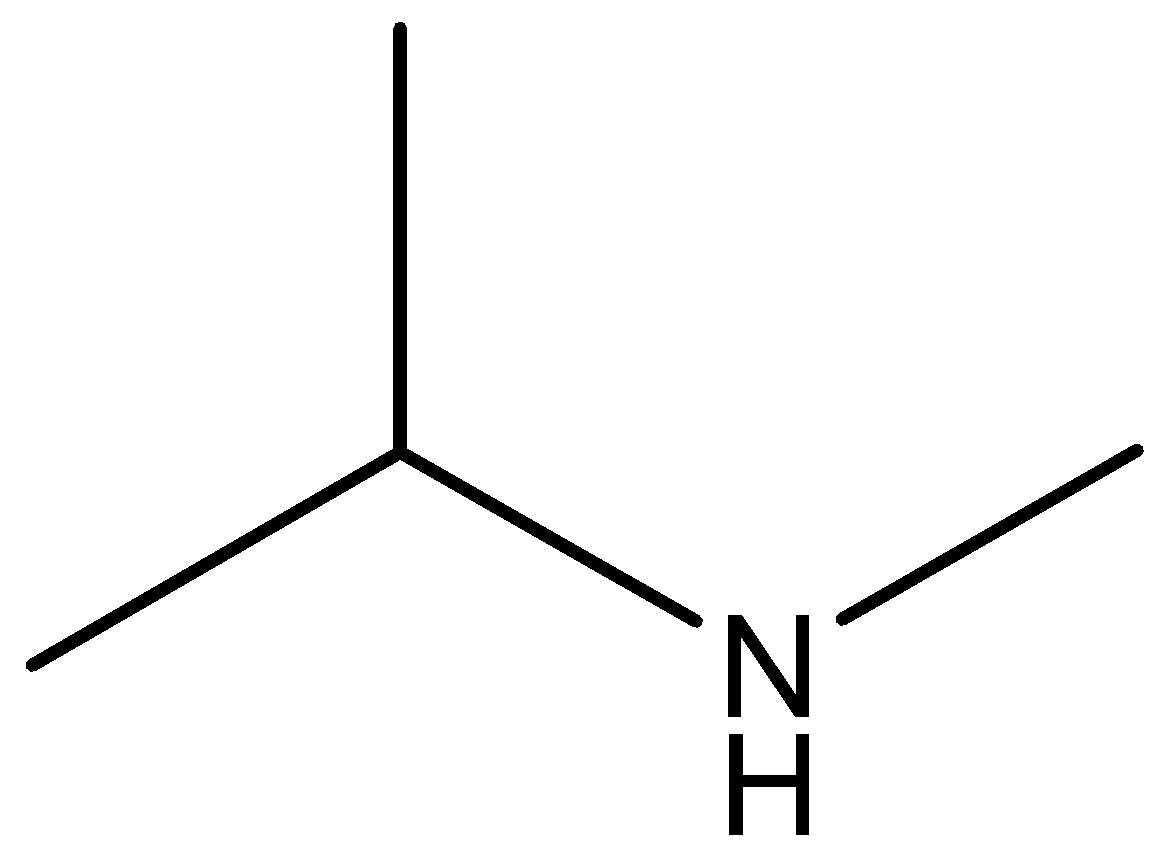
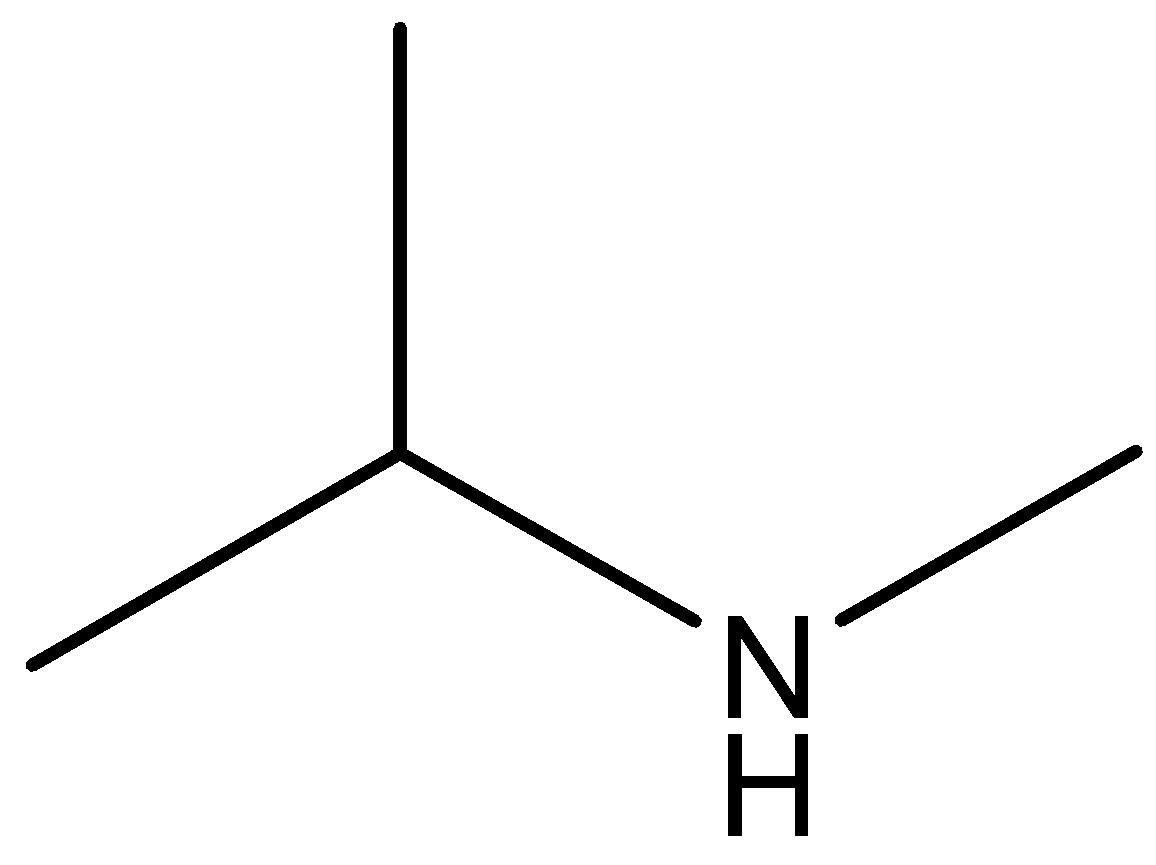
The molecular formula of secondary amine is
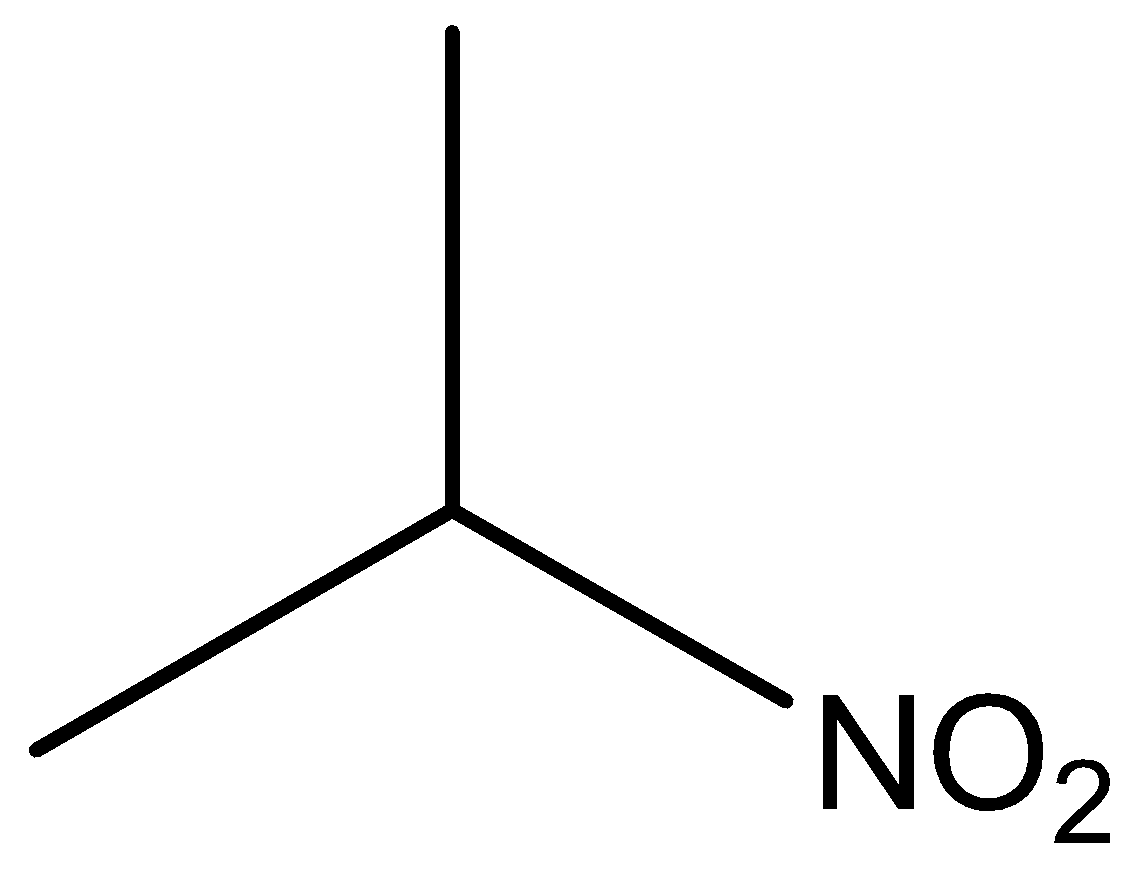
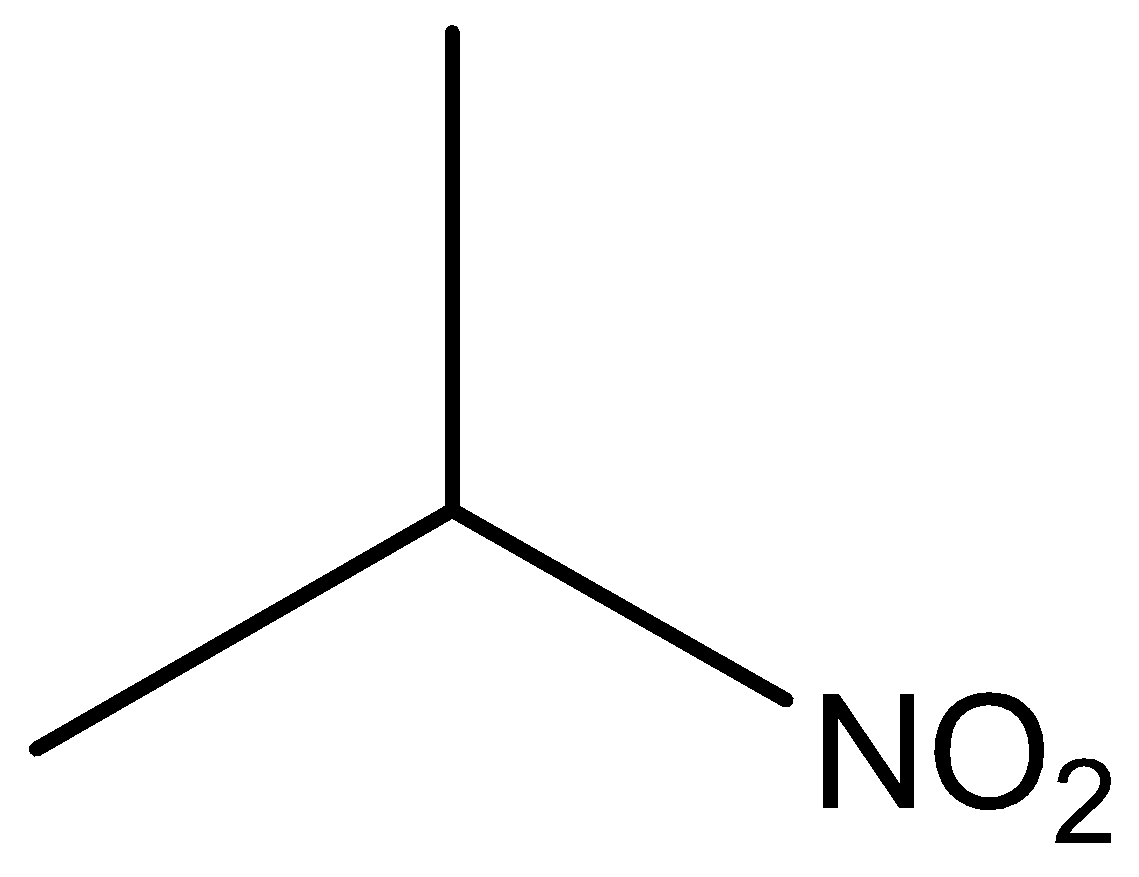
The molecular formula of secondary nitro is
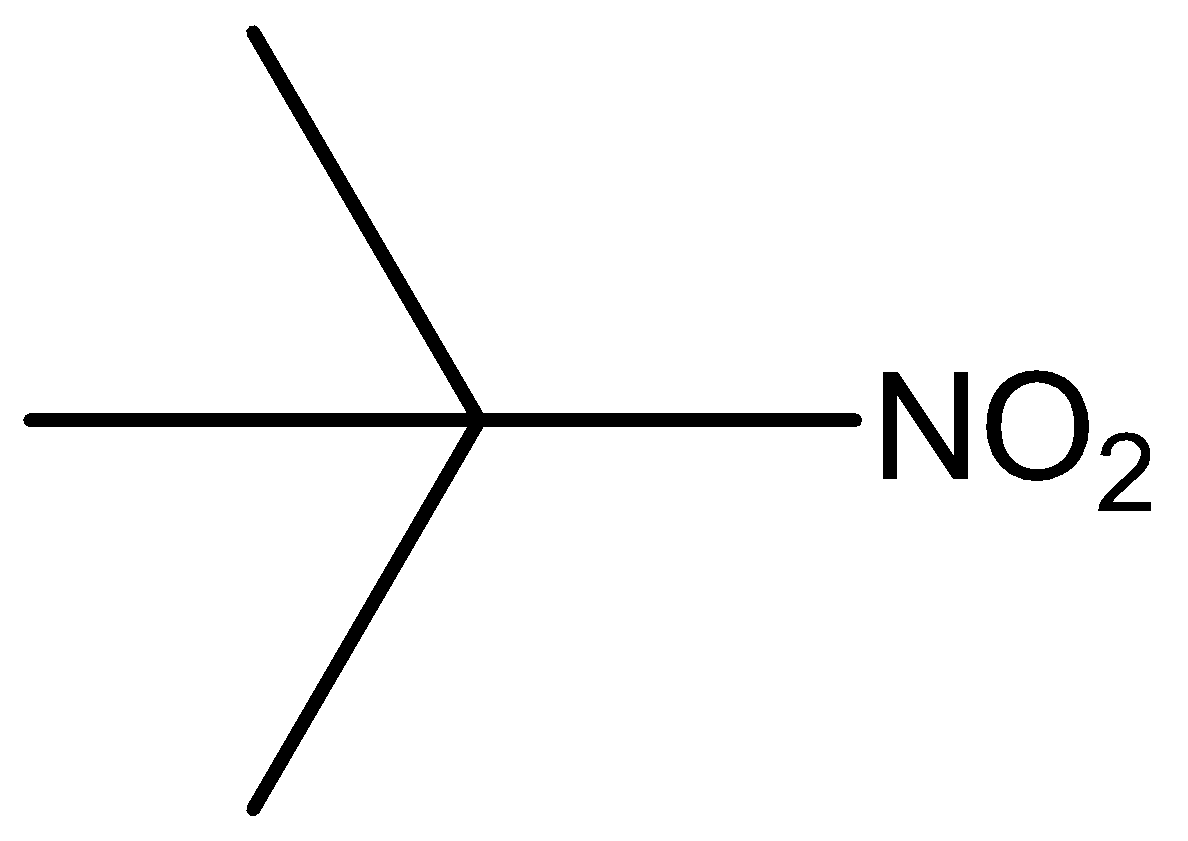
The molecular formula of tertiary nitro is
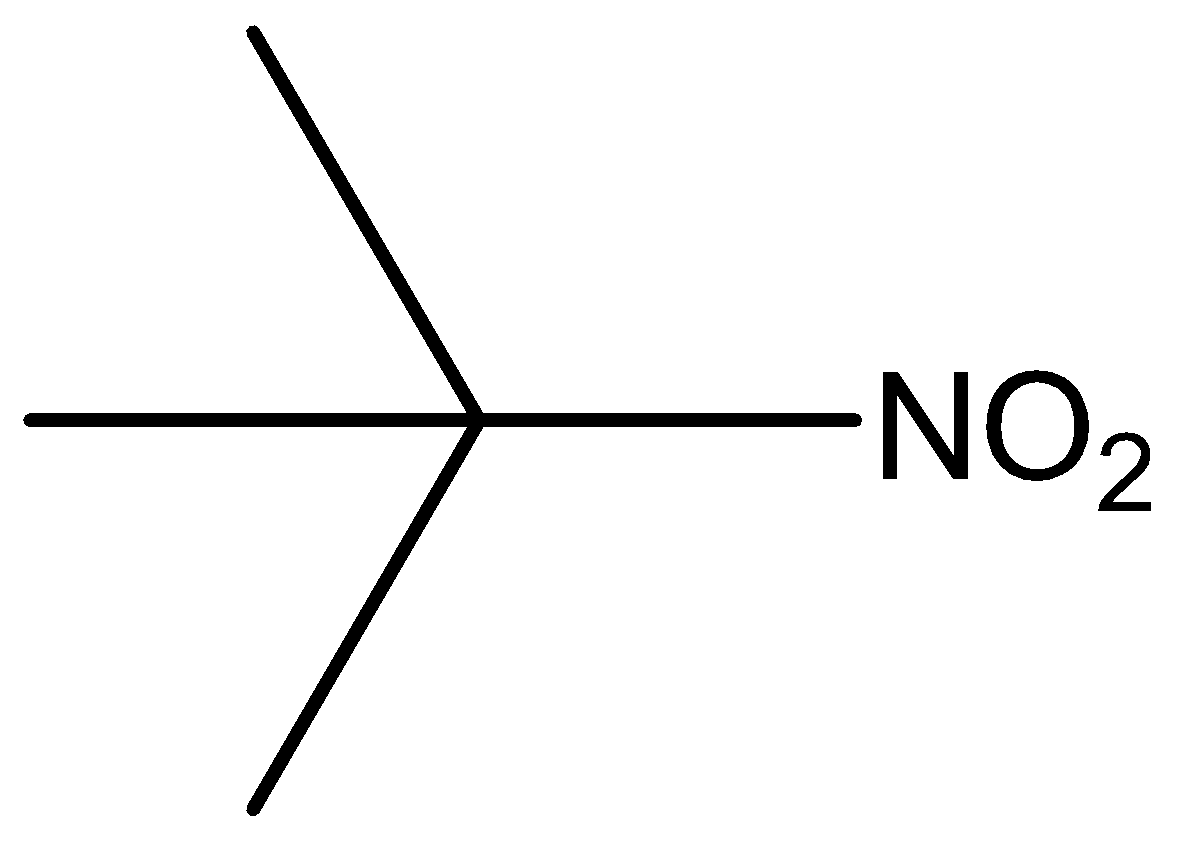
Phenol, secondary amine and secondary nitro are reacted with \[{\text{HN}}{{\text{O}}_{\text{2}}}\]. But tertiary nitro is not with \[{\text{HN}}{{\text{O}}_{\text{2}}}\] because it does not have alpha hydrogen atoms. Phenol, secondary amine and secondary nitro are react with \[{\text{HN}}{{\text{O}}_{\text{2}}}\], because they have alpha hydrogen atom.
According to the above discussion, we conclude tertiary nitro does not react with \[{\text{HN}}{{\text{O}}_{\text{2}}}\].
Hence, option D is the correct answer.
Note:
We must know that secondary amine compounds are more reactive than tertiary amine compounds. Secondary amine compounds are more reactive than primary amine compounds. Secondary nitro compounds are more reactive than tertiary nitro compounds. Secondary nitro compounds are more reactive than primary nitro compounds. Tertiary amine compounds are more reactive than primary amine compounds. Tertiary nitro compounds are more reactive than primary nitro compounds.
Complete answer:
According to the Arrhenius concept, one substance said to be acidic means acid is nothing but a substance that dissociates to give hydrogen ions when decomposed in the water.
Examples of Arrhenius acids are hydrochloric acid (\[{\text{HCl}}\]), sulphuric acid (\[{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\]), nitrous acid (\[{\text{HN}}{{\text{O}}_2}\]) etc,.
Hence, \[{\text{HN}}{{\text{O}}_2}\] is acidic.
The molecular formula of phenol is

The molecular formula of secondary amine is

The molecular formula of secondary nitro is

The molecular formula of tertiary nitro is

Phenol, secondary amine and secondary nitro are reacted with \[{\text{HN}}{{\text{O}}_{\text{2}}}\]. But tertiary nitro is not with \[{\text{HN}}{{\text{O}}_{\text{2}}}\] because it does not have alpha hydrogen atoms. Phenol, secondary amine and secondary nitro are react with \[{\text{HN}}{{\text{O}}_{\text{2}}}\], because they have alpha hydrogen atom.
According to the above discussion, we conclude tertiary nitro does not react with \[{\text{HN}}{{\text{O}}_{\text{2}}}\].
Hence, option D is the correct answer.
Note:
We must know that secondary amine compounds are more reactive than tertiary amine compounds. Secondary amine compounds are more reactive than primary amine compounds. Secondary nitro compounds are more reactive than tertiary nitro compounds. Secondary nitro compounds are more reactive than primary nitro compounds. Tertiary amine compounds are more reactive than primary amine compounds. Tertiary nitro compounds are more reactive than primary nitro compounds.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Class 11 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




