
Which of the following does not have S-S linkage?
a.) \[{{S}_{2}}O_{8}^{2-}\]
b.) \[{{S}_{2}}O_{6}^{2-}\]
c.) \[{{S}_{2}}O_{5}^{2-}\]
d.) \[{{S}_{2}}O_{3}^{2-}\]
Answer
591.3k+ views
Hint: S-S linkage, it means the compound should contain a bond between two sulphur atoms. To know about the S-S linkage we should know the structure of the compounds. The oxides of the sulphur contain S-S linkage.
Complete step by step answer:
All the compounds which are present in the given question are the oxides of sulphur.
The compound in option A is \[{{S}_{2}}O_{8}^{2-}\]. The structure of \[{{S}_{2}}O_{8}^{2-}\] is as follows.
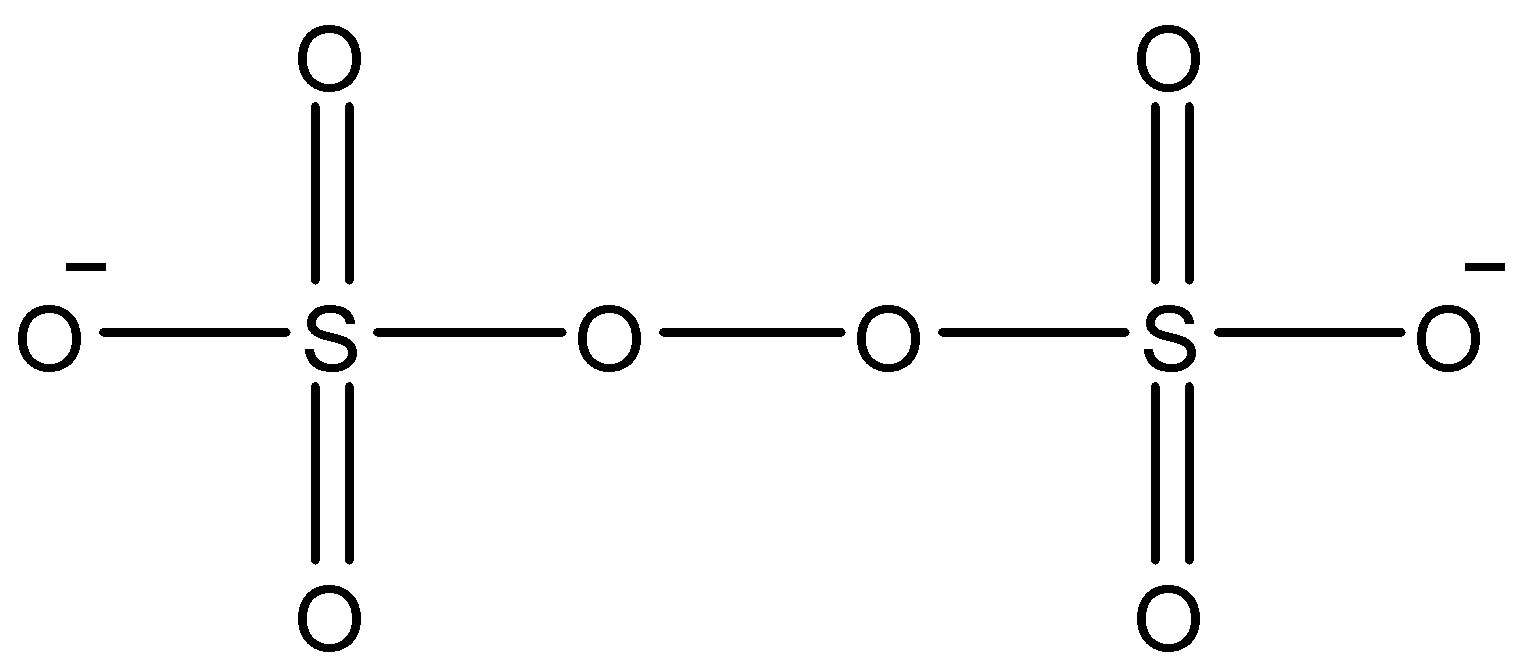
In the above structure we can see that there is no bond between two sulphur atoms (S-S linkage) clearly. So, \[{{S}_{2}}O_{8}^{2-}\]does not contain S-S linkage.
Coming to option B,\[{{S}_{2}}O_{6}^{2-}\]. The structure of \[{{S}_{2}}O_{6}^{2-}\] is as follows
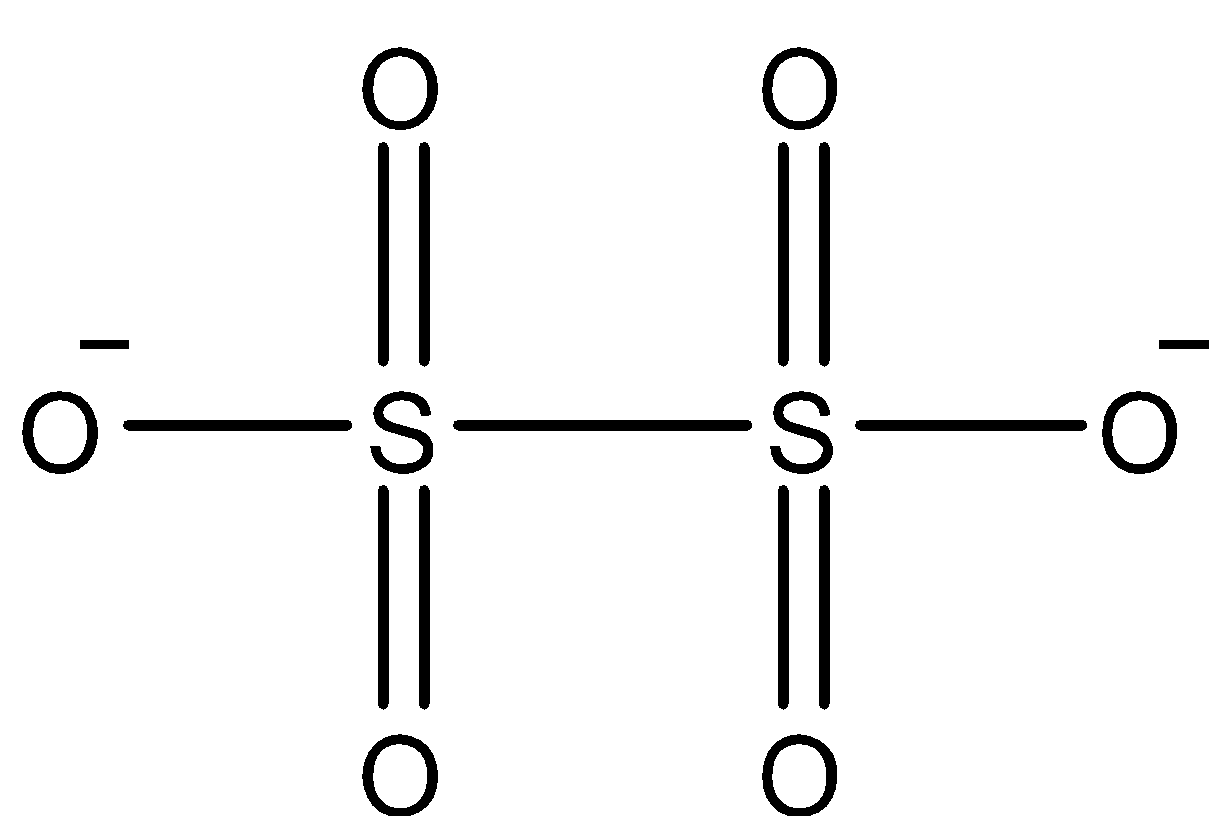
In the above structure there is an S-S linkage (bond between sulphur atoms), we can see it clearly.
Coming to option C, \[{{S}_{2}}O_{5}^{2-}\]. The structure of \[{{S}_{2}}O_{5}^{2-}\]is as follows
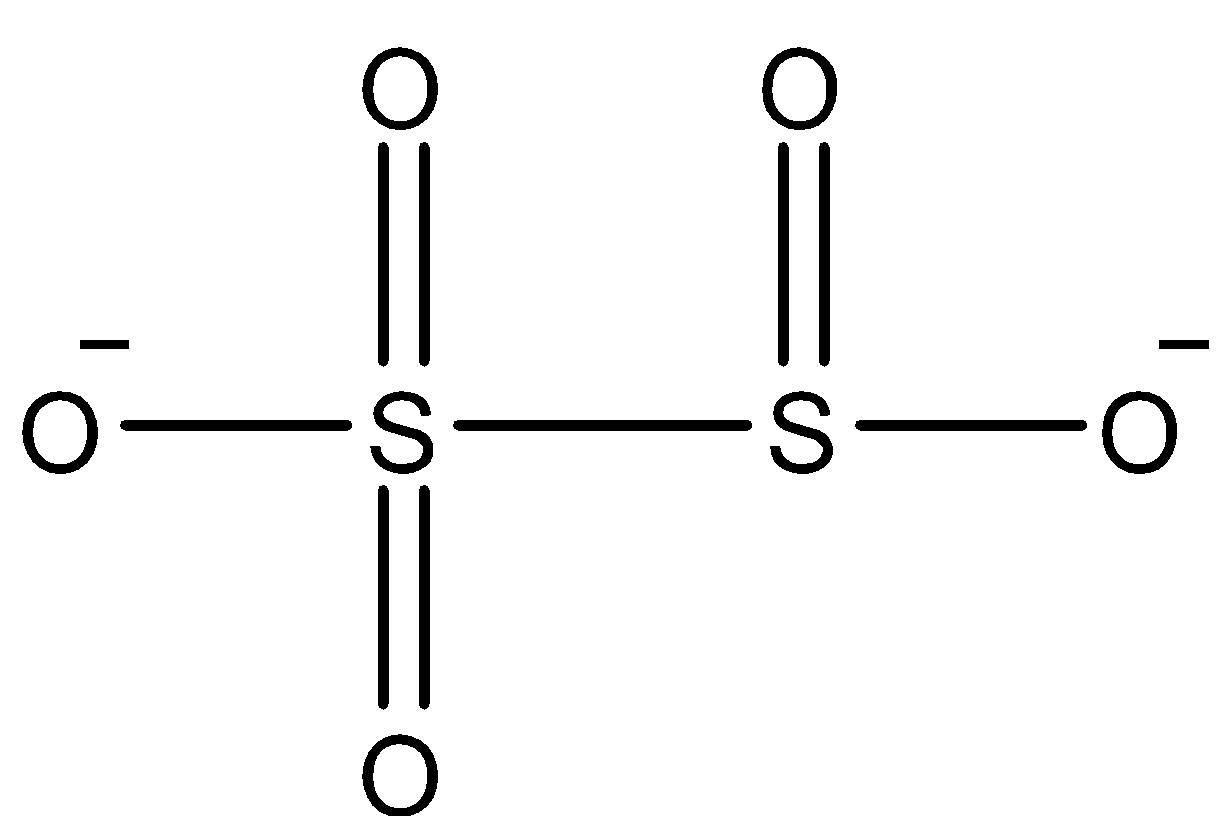
In the above structure there is an S-S linkage (bond between sulphur atoms), we can see it clearly.
Coming to option D, \[{{S}_{2}}O_{3}^{2-}\]. The structure of \[{{S}_{2}}O_{3}^{2-}\] is as follows.
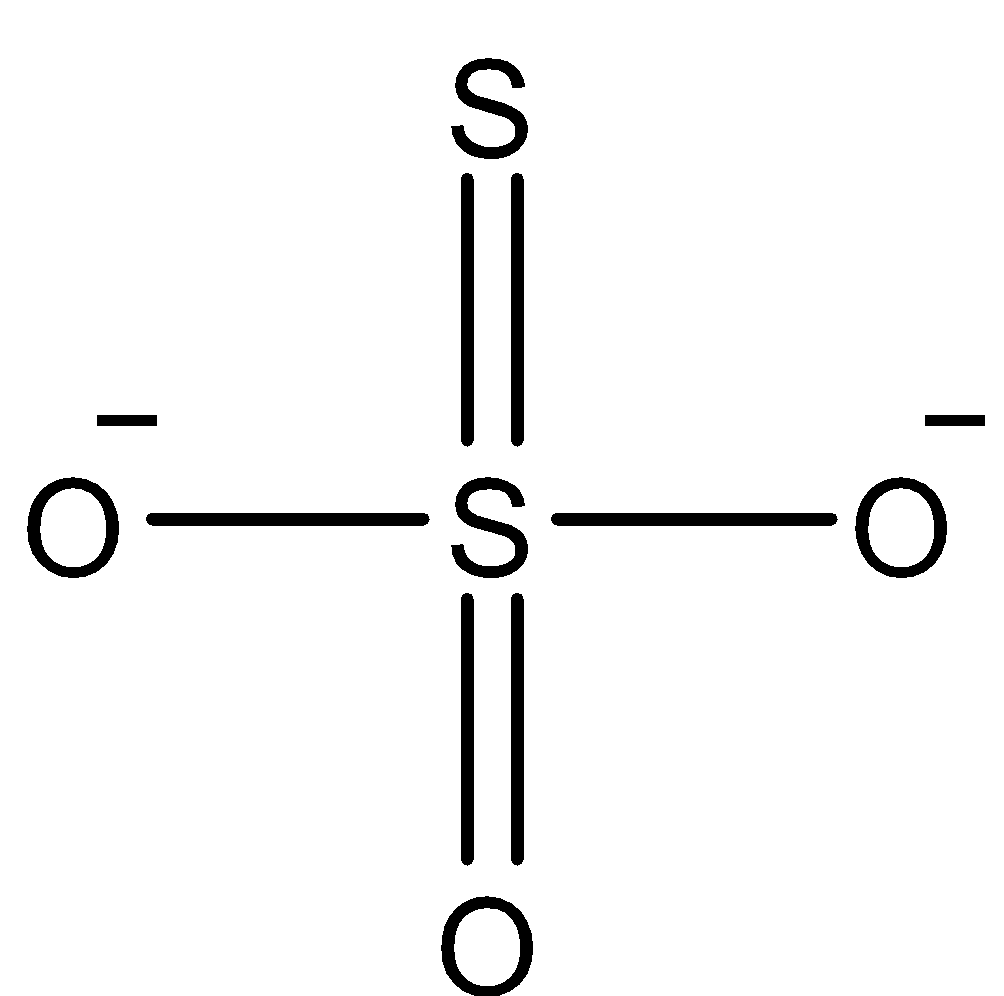
In the above structure there is an S-S linkage (bond between sulphur atoms), we can see it clearly.
Therefore, the compound that does not contain S-S linkage is \[{{S}_{2}}O_{8}^{2-}\].
So, the correct answer is “Option A”.
Note:To know about the presence of S-S linkage in any compound we have to draw the structure of the compounds. If we are not going to draw the structures then we cannot say the presence of S-S linkage.
Complete step by step answer:
All the compounds which are present in the given question are the oxides of sulphur.
The compound in option A is \[{{S}_{2}}O_{8}^{2-}\]. The structure of \[{{S}_{2}}O_{8}^{2-}\] is as follows.

In the above structure we can see that there is no bond between two sulphur atoms (S-S linkage) clearly. So, \[{{S}_{2}}O_{8}^{2-}\]does not contain S-S linkage.
Coming to option B,\[{{S}_{2}}O_{6}^{2-}\]. The structure of \[{{S}_{2}}O_{6}^{2-}\] is as follows

In the above structure there is an S-S linkage (bond between sulphur atoms), we can see it clearly.
Coming to option C, \[{{S}_{2}}O_{5}^{2-}\]. The structure of \[{{S}_{2}}O_{5}^{2-}\]is as follows

In the above structure there is an S-S linkage (bond between sulphur atoms), we can see it clearly.
Coming to option D, \[{{S}_{2}}O_{3}^{2-}\]. The structure of \[{{S}_{2}}O_{3}^{2-}\] is as follows.

In the above structure there is an S-S linkage (bond between sulphur atoms), we can see it clearly.
Therefore, the compound that does not contain S-S linkage is \[{{S}_{2}}O_{8}^{2-}\].
So, the correct answer is “Option A”.
Note:To know about the presence of S-S linkage in any compound we have to draw the structure of the compounds. If we are not going to draw the structures then we cannot say the presence of S-S linkage.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




