
Which of the following does not have ${{s}}{{{p}}^2}$ hybridization?
A. ${{C}}{ _6}{{{H}}_6}$
B. ${{{C}}_2}{{{H}}_4}$
C. ${{BC}}{{{l}}_3}$
D. ${{{C}}_2}{{{H}}_2}$
Answer
558.6k+ views
Hint: Let’s know about hybridization first. When we combine two or more atomic orbitals having similar energies, we get a new form of orbitals which are called hybrid orbitals. This phenomena is called hybridization.
Complete step by step answer:
We know that compounds having double bonds will have ${{s}}{{{p}}^2}$ hybridization. Double bond indicates that they will have a $\sigma $ bond and $\pi $ bond. While single bonded compounds have ${{s}}{{{p}}^3}$ hybridization. Single bond indicates that it has only $\sigma $ bond. Triple bonded compounds have ${{sp}}$ hybridization. It has one $\sigma $ bond and two $\pi $ bonds. $\sigma $ bond can be formed independent of any other bond between two atoms. While $\pi $ bond can be formed only if there is a $\sigma $ bond already formed.
Now let’s focus on the structures of these compounds to know more about hybridization.
A. The structure of ${{C}}{ _6}{{{H}}_6}$ is given below:
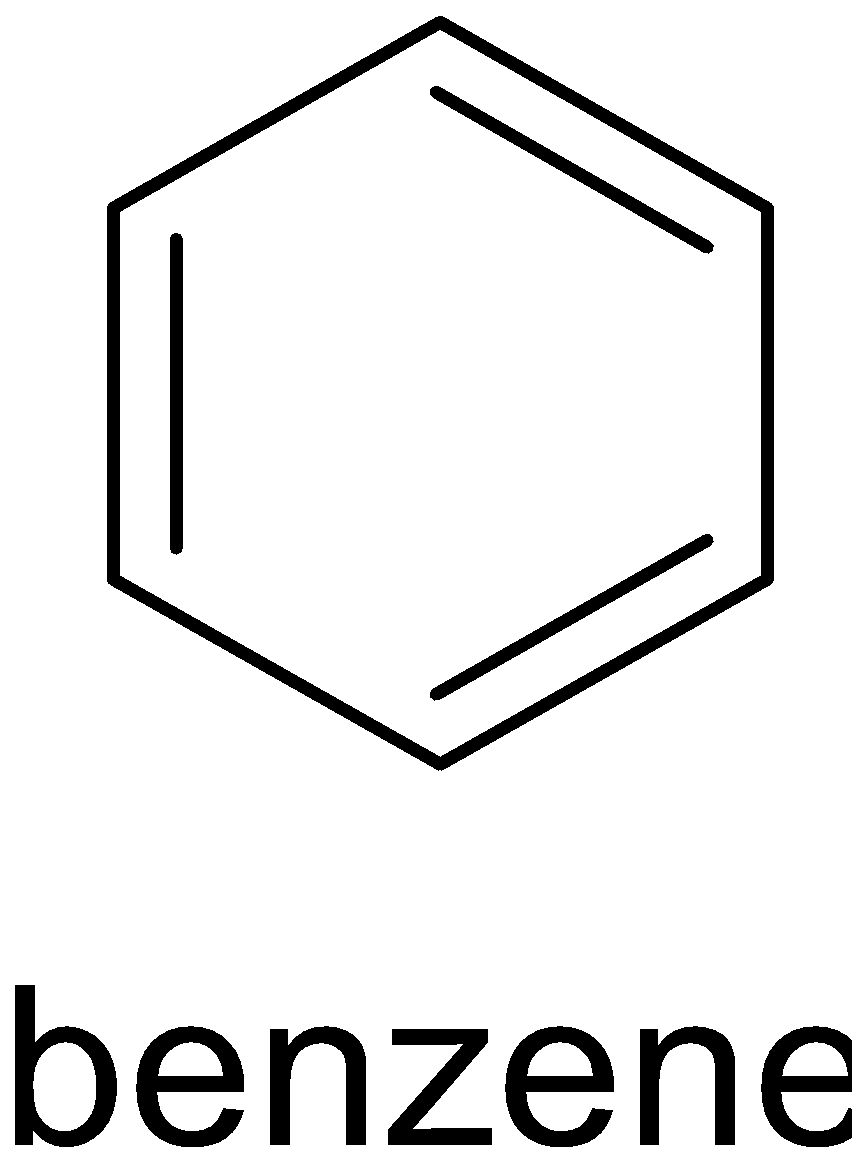
It has three $\pi $ bonds. Thus all of the carbon atoms are ${{s}}{{{p}}^2}$ hybridized.
B. The structure of ${{{C}}_2}{{{H}}_4}$ is given below:
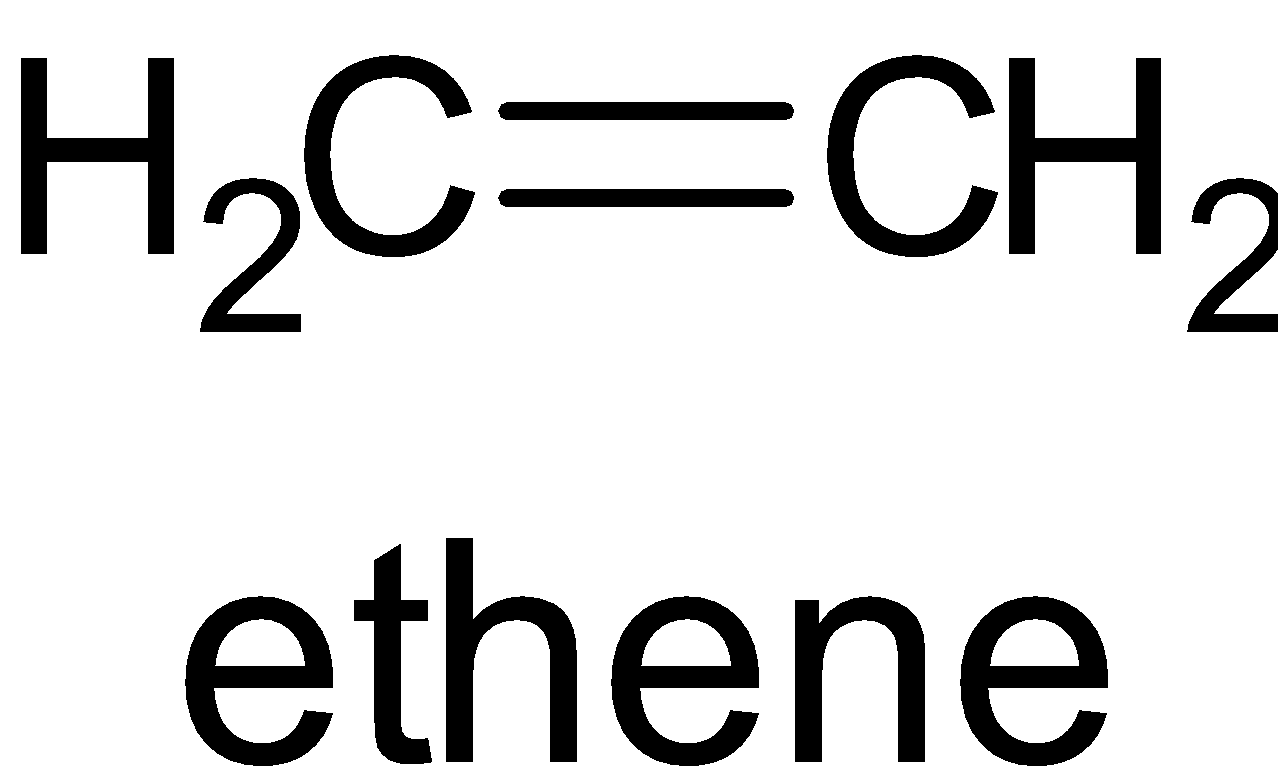
In this molecule, there is one $\sigma $ bond and $\pi $ bond. Thus both of the carbon atoms are ${{s}}{{{p}}^2}$ hybridized.
C. The structure is given below:
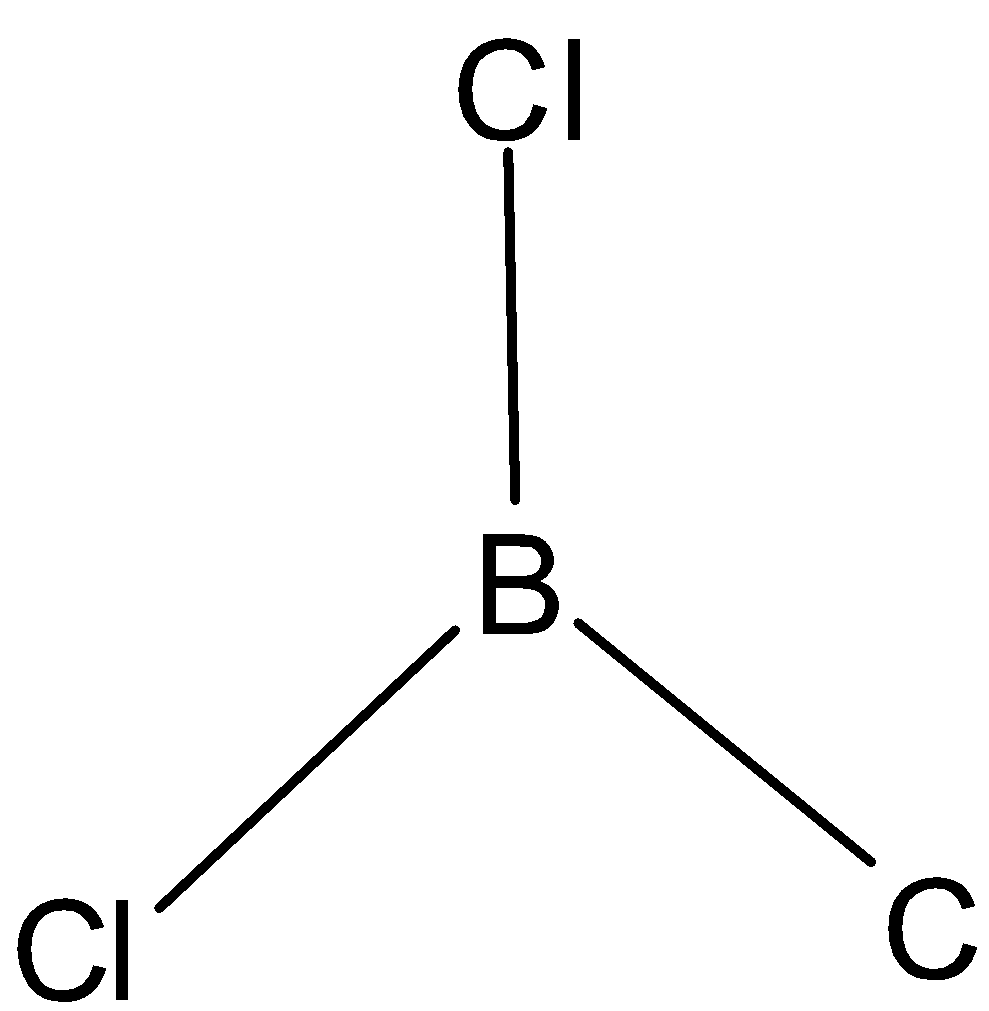
The hybridization in this molecule is determined using steric formula. The central atom is boron. Three chlorine atoms are attached to boron atoms. Steric number is obtained when we add the number of atoms bonded to the central atom and lone pairs. Here, there are no lone pairs. Thus steric number is three. Thus it has ${{s}}{{{p}}^2}$ hybridization.
D. The structure is given below:
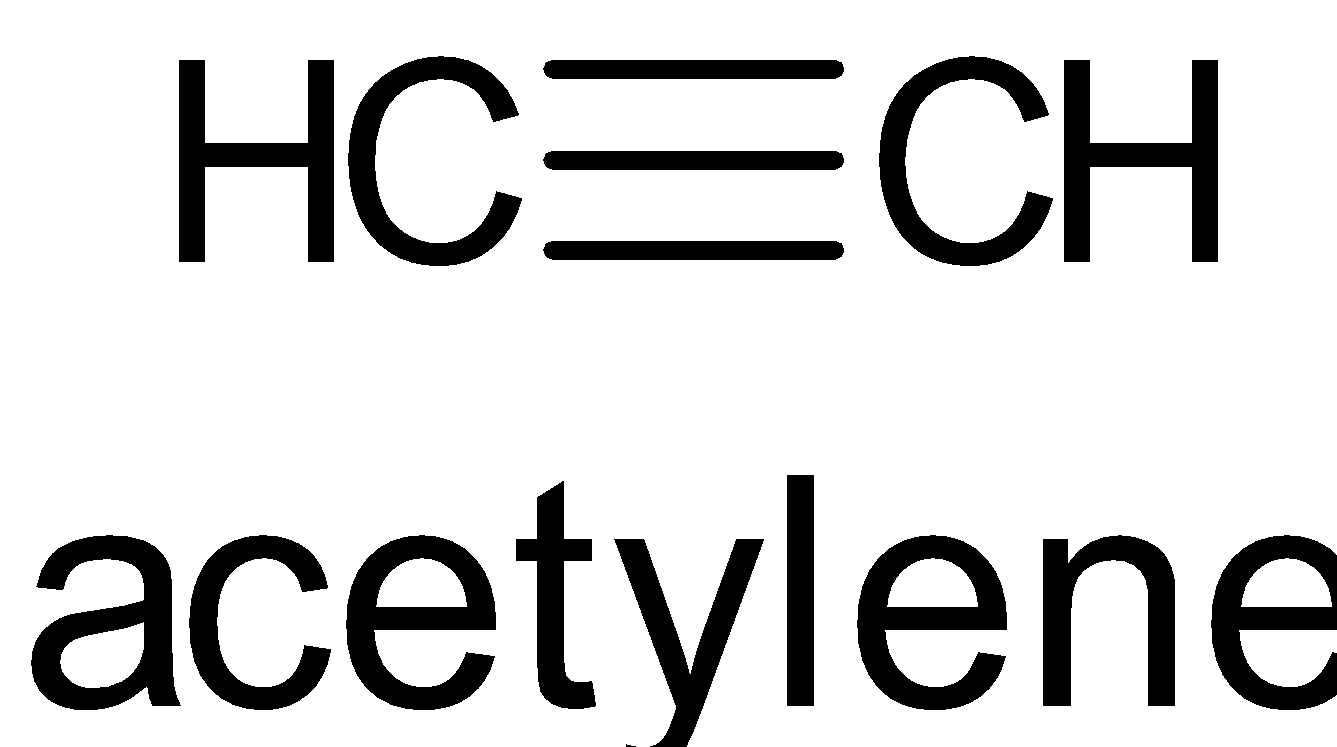
It has one $\sigma $ bond and two $\pi $ bonds. It has ${{sp}}$ hybridization.
So ${{BC}}{{{l}}_3}$ and ${{{C}}_2}{{{H}}_2}$ does not have ${{sp}}$ hybridization.
So, the correct answer is Option D.
Note: Molecular geometry can be determined from the hybridization. Steric number shows the arrangement of electron pairs thereby we obtain the geometry. This attains a geometry so that the distance between the electron pairs are maximum. This is based on the VSEPR theory, i.e. valence shell electron pair repulsion theory.
Complete step by step answer:
We know that compounds having double bonds will have ${{s}}{{{p}}^2}$ hybridization. Double bond indicates that they will have a $\sigma $ bond and $\pi $ bond. While single bonded compounds have ${{s}}{{{p}}^3}$ hybridization. Single bond indicates that it has only $\sigma $ bond. Triple bonded compounds have ${{sp}}$ hybridization. It has one $\sigma $ bond and two $\pi $ bonds. $\sigma $ bond can be formed independent of any other bond between two atoms. While $\pi $ bond can be formed only if there is a $\sigma $ bond already formed.
Now let’s focus on the structures of these compounds to know more about hybridization.
A. The structure of ${{C}}{ _6}{{{H}}_6}$ is given below:

It has three $\pi $ bonds. Thus all of the carbon atoms are ${{s}}{{{p}}^2}$ hybridized.
B. The structure of ${{{C}}_2}{{{H}}_4}$ is given below:
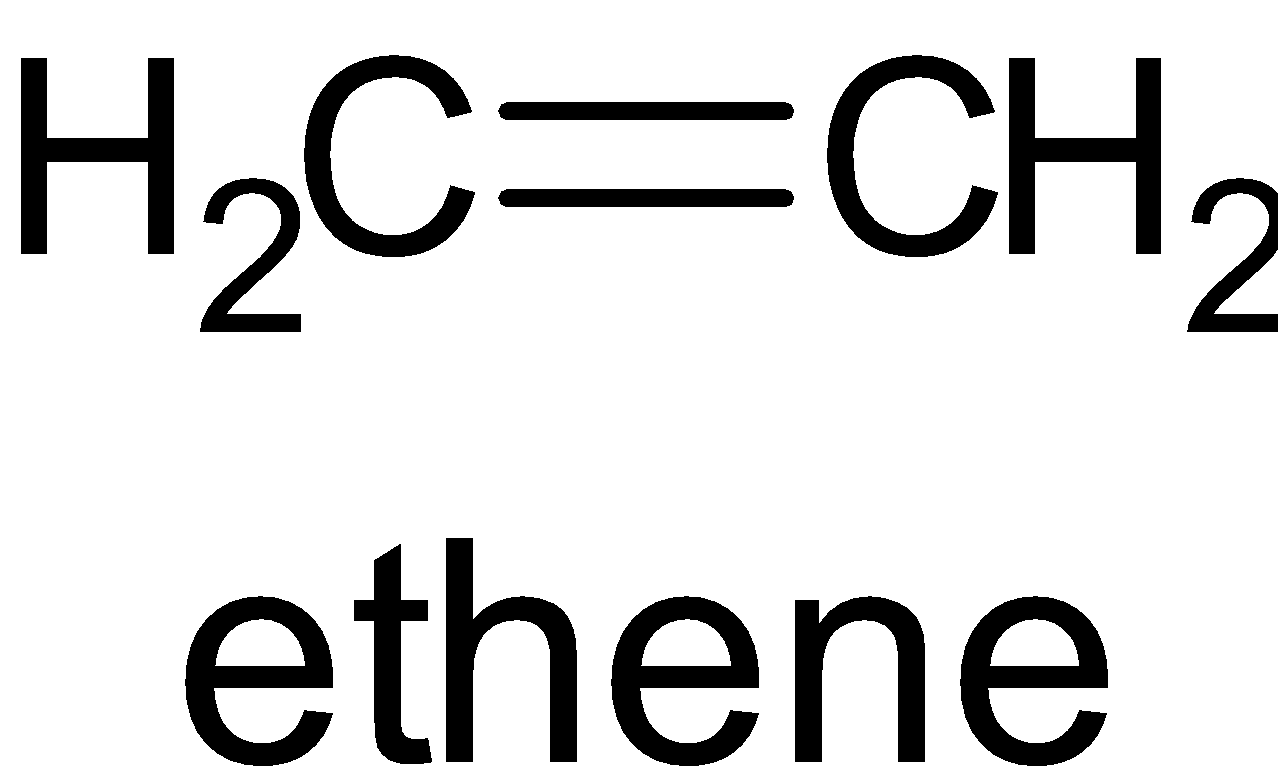
In this molecule, there is one $\sigma $ bond and $\pi $ bond. Thus both of the carbon atoms are ${{s}}{{{p}}^2}$ hybridized.
C. The structure is given below:

The hybridization in this molecule is determined using steric formula. The central atom is boron. Three chlorine atoms are attached to boron atoms. Steric number is obtained when we add the number of atoms bonded to the central atom and lone pairs. Here, there are no lone pairs. Thus steric number is three. Thus it has ${{s}}{{{p}}^2}$ hybridization.
D. The structure is given below:
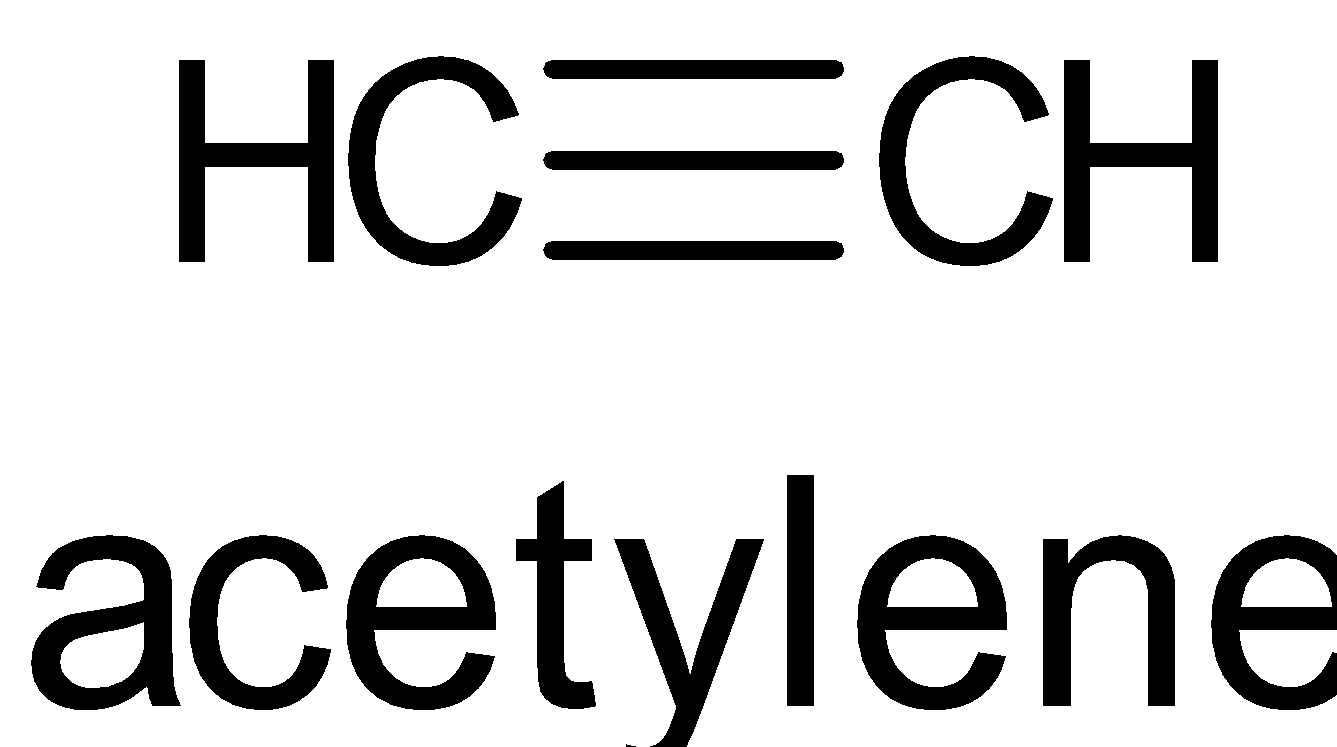
It has one $\sigma $ bond and two $\pi $ bonds. It has ${{sp}}$ hybridization.
So ${{BC}}{{{l}}_3}$ and ${{{C}}_2}{{{H}}_2}$ does not have ${{sp}}$ hybridization.
So, the correct answer is Option D.
Note: Molecular geometry can be determined from the hybridization. Steric number shows the arrangement of electron pairs thereby we obtain the geometry. This attains a geometry so that the distance between the electron pairs are maximum. This is based on the VSEPR theory, i.e. valence shell electron pair repulsion theory.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




