
Which of the following does not depict properties of fullerenes?
A) Fullerenes are made by heating graphite.
B) Fullerenes are pure forms of carbon.
C) Fullerenes have open cage structure like ice.
D) \[{{\text{C}}_{60}}\] is called Buckminsterfullerene.
Answer
566.7k+ views
Hint: The fullerenes are allotropes of carbon. They have cage like appearance and soccer ball shape or football shape. They only contain carbon atoms. They do not contain impurities.
Complete step-by-step answer:
Fullerenes are spherical in shape and have composition of \[{{\text{C}}_{2n}}\] where \[n \geqslant 30\] . wecan dissolve fullerene in organic solvents. In fullerene, the carbon atom undergoes \[s{p^2}\] hybridization. To prepare fullerenes we heat graphite in an electric arc. We provide an atmosphere of inert gases helium or argon. wecan also evaporate graphite with a laser to prepare fullerenes. Thus, the statement of the option (A) depicts properties of fullerenes.
Fullerenes, graphite and diamond are pure forms of carbon. They all are carbon allotropes. They contain only carbon atoms. They do not contain impurities. Thus, the statement of the option (B) depicts properties of fullerenes.
\[{{\text{C}}_{60}}\] is called Buckminsterfullerene as it has a structure similar to geodesic domes. Thus, the statement of the option (D) depicts properties of fullerenes.
Fullerenes have a closed cage structure like a soccer ball. The following does not depict properties of
fullerenes. Fullerenes have open cage structure like ice. Hence, the correct option is the option (C).
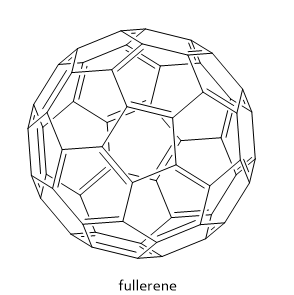
Note: Different fullerenes have different numbers of hexagons, and have different molecular sizes. For example, \[{{\text{C}}_{60}}\] molecule has 60 carbon atoms. These carbon atoms are arranged as 12 pentagons and 20 hexagons. Some other examples of fullerenes include \[{{\text{C}}_{70}},{{\text{C}}_{28}},{{\text{C}}_{36}},{{\text{C}}_{50}}\] molecules.
Complete step-by-step answer:
Fullerenes are spherical in shape and have composition of \[{{\text{C}}_{2n}}\] where \[n \geqslant 30\] . wecan dissolve fullerene in organic solvents. In fullerene, the carbon atom undergoes \[s{p^2}\] hybridization. To prepare fullerenes we heat graphite in an electric arc. We provide an atmosphere of inert gases helium or argon. wecan also evaporate graphite with a laser to prepare fullerenes. Thus, the statement of the option (A) depicts properties of fullerenes.
Fullerenes, graphite and diamond are pure forms of carbon. They all are carbon allotropes. They contain only carbon atoms. They do not contain impurities. Thus, the statement of the option (B) depicts properties of fullerenes.
\[{{\text{C}}_{60}}\] is called Buckminsterfullerene as it has a structure similar to geodesic domes. Thus, the statement of the option (D) depicts properties of fullerenes.
Fullerenes have a closed cage structure like a soccer ball. The following does not depict properties of
fullerenes. Fullerenes have open cage structure like ice. Hence, the correct option is the option (C).

Note: Different fullerenes have different numbers of hexagons, and have different molecular sizes. For example, \[{{\text{C}}_{60}}\] molecule has 60 carbon atoms. These carbon atoms are arranged as 12 pentagons and 20 hexagons. Some other examples of fullerenes include \[{{\text{C}}_{70}},{{\text{C}}_{28}},{{\text{C}}_{36}},{{\text{C}}_{50}}\] molecules.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




