
Which of the following does not apply to metallic bonds?
(a)- Overlapping Valence Orbitals
(b)- Mobile Valence Electron
(c)- Delocalized Electron
(d)- Highly Directed Bond
Answer
516.6k+ views
Hint: Metallic bond is formed by the kernels of the metal or ions of the metal and electrons surrounding them. The electrons are surrounded by the kernel in all directions and the electrons surrounding the metal ions.
Complete answer:
First, let us see what metallic bond is formed. So, the metallic bond is formed between kernels of the metal and the delocalized electrons surrounding the kernels. Kernels of the metal are also known as metal ions. This is shown below:
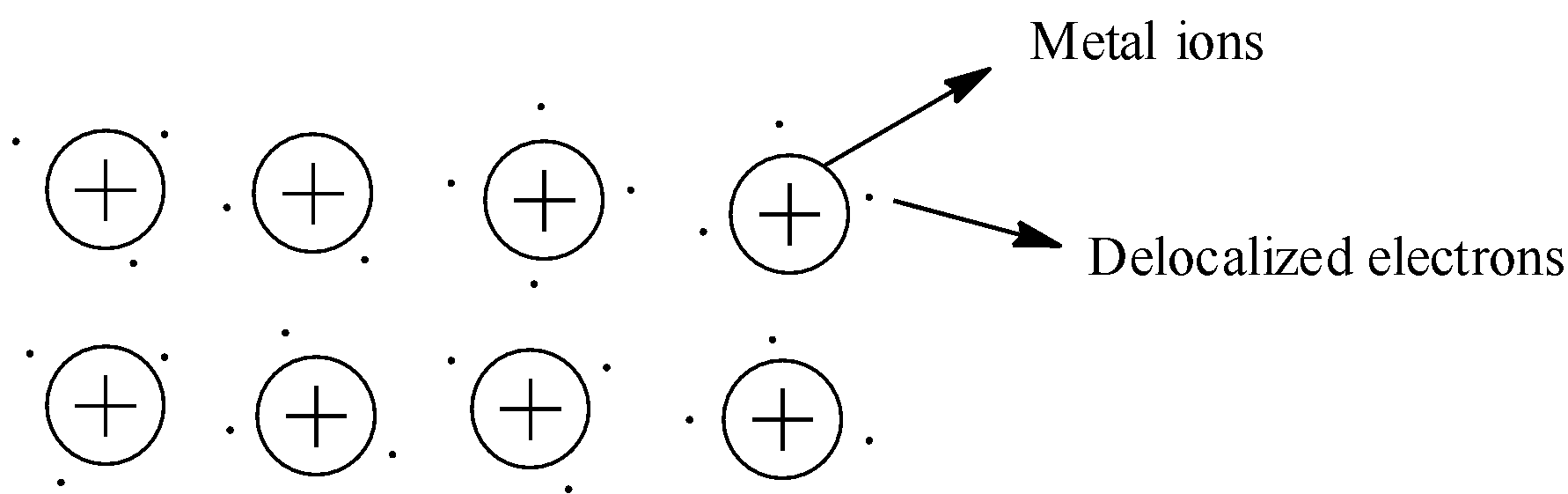
So, the electrons surrounding the kernels are in all directions. These electrons and metal ions have electrostatic forces. Since the electrons are surrounded in all the directions to the kernel, the electrostatic forces are in all the directions of the metal ion.
So, there is overlapping of the valence orbitals because without overlapping of orbitals no bond can form.
Since the electrons are delocalized, therefore, the electrons can be moved from one place to another. Since the force is in all directions we can say that the bond is not directed in any direction.
Therefore, from all the options (a), (b), and (c) can be true for the metallic bond.
Hence, the correct answer is option (d)- Highly Directed Bond.
Note:
Some of the bonds which have directions or which are called directional bonds are covalent bonds, coordinate bonds, etc. These are formed by the directions of overlapping of orbitals, movement of electrons, etc.
Complete answer:
First, let us see what metallic bond is formed. So, the metallic bond is formed between kernels of the metal and the delocalized electrons surrounding the kernels. Kernels of the metal are also known as metal ions. This is shown below:

So, the electrons surrounding the kernels are in all directions. These electrons and metal ions have electrostatic forces. Since the electrons are surrounded in all the directions to the kernel, the electrostatic forces are in all the directions of the metal ion.
So, there is overlapping of the valence orbitals because without overlapping of orbitals no bond can form.
Since the electrons are delocalized, therefore, the electrons can be moved from one place to another. Since the force is in all directions we can say that the bond is not directed in any direction.
Therefore, from all the options (a), (b), and (c) can be true for the metallic bond.
Hence, the correct answer is option (d)- Highly Directed Bond.
Note:
Some of the bonds which have directions or which are called directional bonds are covalent bonds, coordinate bonds, etc. These are formed by the directions of overlapping of orbitals, movement of electrons, etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




