
Which of the following describes the bonds in $PC{l_5}$?
A. 2 axial and 3 equatorial
B. 3 axial and 2 equatorial
C. 4 axial and 5 equatorial
D. 5 axial and 4 equatorial
Answer
507k+ views
Hint: We have to first predict the geometry of the compound to describe the bonds. We can use VSEPR theory to predict the geometry of the compound. We have to first predict the steric number using the bond pairs that are bonded to the central atom and the number of lone pairs present in the central atom.
Complete answer:
In $PC{l_5}$, five atoms of chlorine are bonded to one atom of phosphorus. Let us now draw the Lewis structure of $PC{l_5}$. In order to draw the Lewis structure, we first need to calculate the total number of valence electrons in $PC{l_5}$.
The total number of valence electrons in phosphorus is five and for five chlorine atoms, the total number of valence electrons is thirty-five. So, the total number of valence electrons in $PC{l_5}$ is forty electrons. We can draw the Lewis structure as,
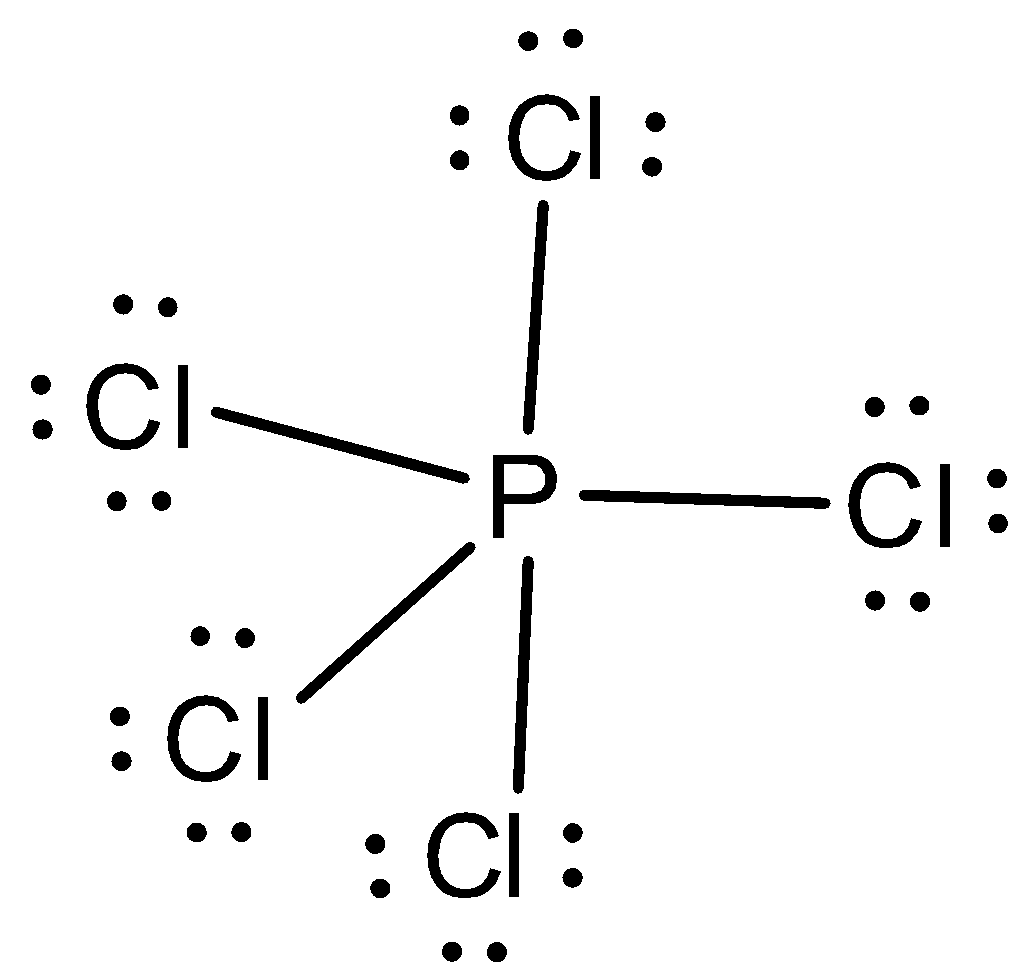
Let us now predict the steric number.
From the Lewis structure, we can see that the central atom has no lone and there are five bond pairs in the central atom. So, the steric number is five. When the steric number is five with no lone pairs in the central atom, the hybridization is $s{p^3}d$ and the molecular geometry is trigonal bipyramidal and the electron pair geometry is also trigonal bipyramidal. The bond angles are $90^\circ ,120^\circ $. There are three equatorial bonds and two axial bonds.
So, the correct answer is “Option A”.
Note:
We have to know that in $PC{l_5}$, the axial bonds are longer when compared to equatorial bonds because of the greater repulsion from equatorial bonds. Hence, axial bonds are closer to equatorial bonds and this leads to more repulsion leading to bond length elongation.
Complete answer:
In $PC{l_5}$, five atoms of chlorine are bonded to one atom of phosphorus. Let us now draw the Lewis structure of $PC{l_5}$. In order to draw the Lewis structure, we first need to calculate the total number of valence electrons in $PC{l_5}$.
The total number of valence electrons in phosphorus is five and for five chlorine atoms, the total number of valence electrons is thirty-five. So, the total number of valence electrons in $PC{l_5}$ is forty electrons. We can draw the Lewis structure as,

Let us now predict the steric number.
From the Lewis structure, we can see that the central atom has no lone and there are five bond pairs in the central atom. So, the steric number is five. When the steric number is five with no lone pairs in the central atom, the hybridization is $s{p^3}d$ and the molecular geometry is trigonal bipyramidal and the electron pair geometry is also trigonal bipyramidal. The bond angles are $90^\circ ,120^\circ $. There are three equatorial bonds and two axial bonds.
So, the correct answer is “Option A”.
Note:
We have to know that in $PC{l_5}$, the axial bonds are longer when compared to equatorial bonds because of the greater repulsion from equatorial bonds. Hence, axial bonds are closer to equatorial bonds and this leads to more repulsion leading to bond length elongation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




