
Which of the following describes the best relationship between the methyl groups in the chair conformation of the substance shown below?
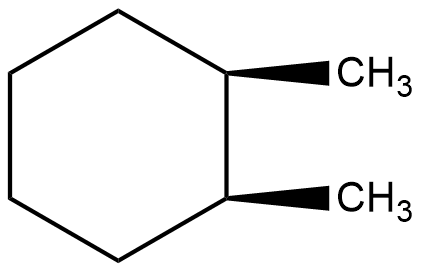
A. Trans
B. Anti
C. Gauche
D. Eclipsed

Answer
513k+ views
Hint: Generally, six membered cyclic organic compounds are going to show high stability in their chair confirmation form. The substituents on the carbon atoms are going to exit in axial or equatorial positions.
Complete step by step answer:
- In the question it is asked to find the best relationship between the methyl groups in the chair conformation of the given compound.
- The Chair form of 1,2-dimethyl cyclohexane is as follows.
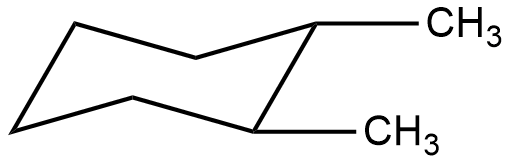
- In the chair form the two methyl groups are cis to each other as per the above structure.
- We can write the conformation for the 1,2-dimethyl cyclohexane and by using the confirmation we can find the relationship between the methyl groups in the chair conformation of the given compound.
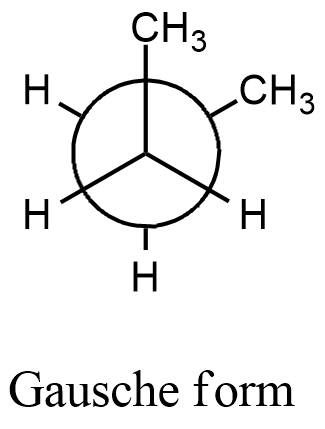
- From the above confirmation we can say that the two methyl groups are going to have an angle of 60 degrees.
- In eclipsed conformation of 1,2-dimethyl cyclohexane the methyl groups are going to overlap one on another.
- Means the gauche form is a more stable conformation for 1,2-dimethyl cyclohexane.
So, the correct option is C.
Note:
In confirmations of the molecules always the gauche form is more stable than the remaining staggered and eclipsed conformations. The reason behind the high stability of the gauche form is due to the less torsional angle between the bulky groups.
Complete step by step answer:
- In the question it is asked to find the best relationship between the methyl groups in the chair conformation of the given compound.
- The Chair form of 1,2-dimethyl cyclohexane is as follows.

- In the chair form the two methyl groups are cis to each other as per the above structure.
- We can write the conformation for the 1,2-dimethyl cyclohexane and by using the confirmation we can find the relationship between the methyl groups in the chair conformation of the given compound.

- From the above confirmation we can say that the two methyl groups are going to have an angle of 60 degrees.
- In eclipsed conformation of 1,2-dimethyl cyclohexane the methyl groups are going to overlap one on another.
- Means the gauche form is a more stable conformation for 1,2-dimethyl cyclohexane.
So, the correct option is C.
Note:
In confirmations of the molecules always the gauche form is more stable than the remaining staggered and eclipsed conformations. The reason behind the high stability of the gauche form is due to the less torsional angle between the bulky groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




