
Which of the following curve explains the Gay Lussac’s law of ideal gases?
A.
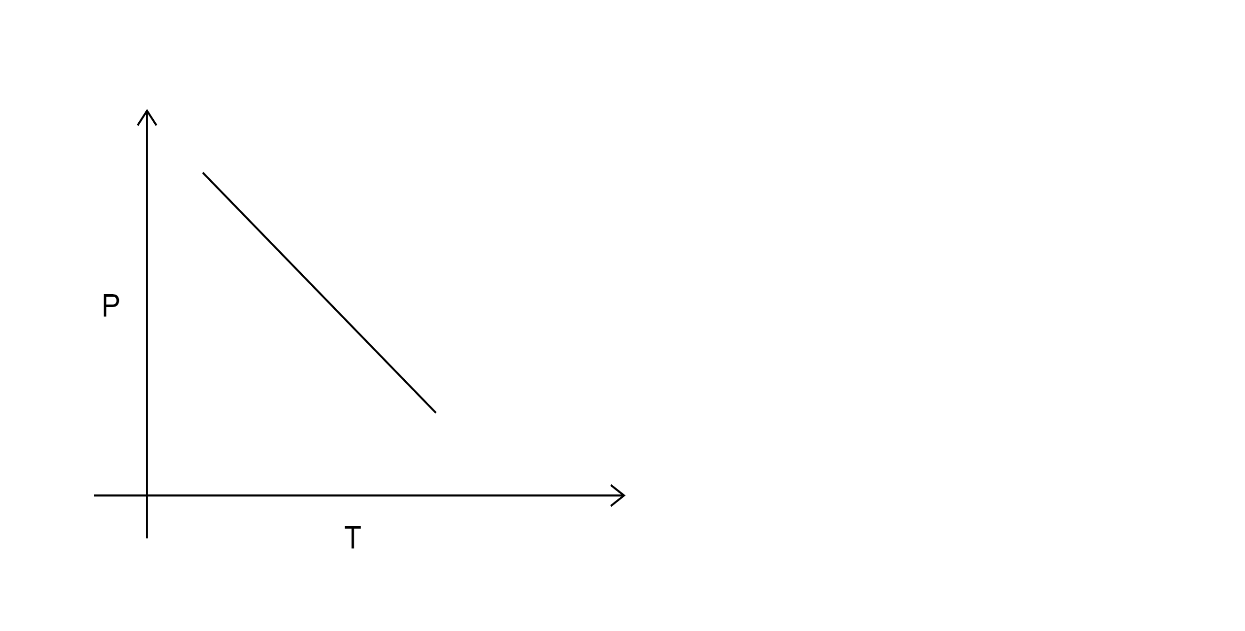
B.
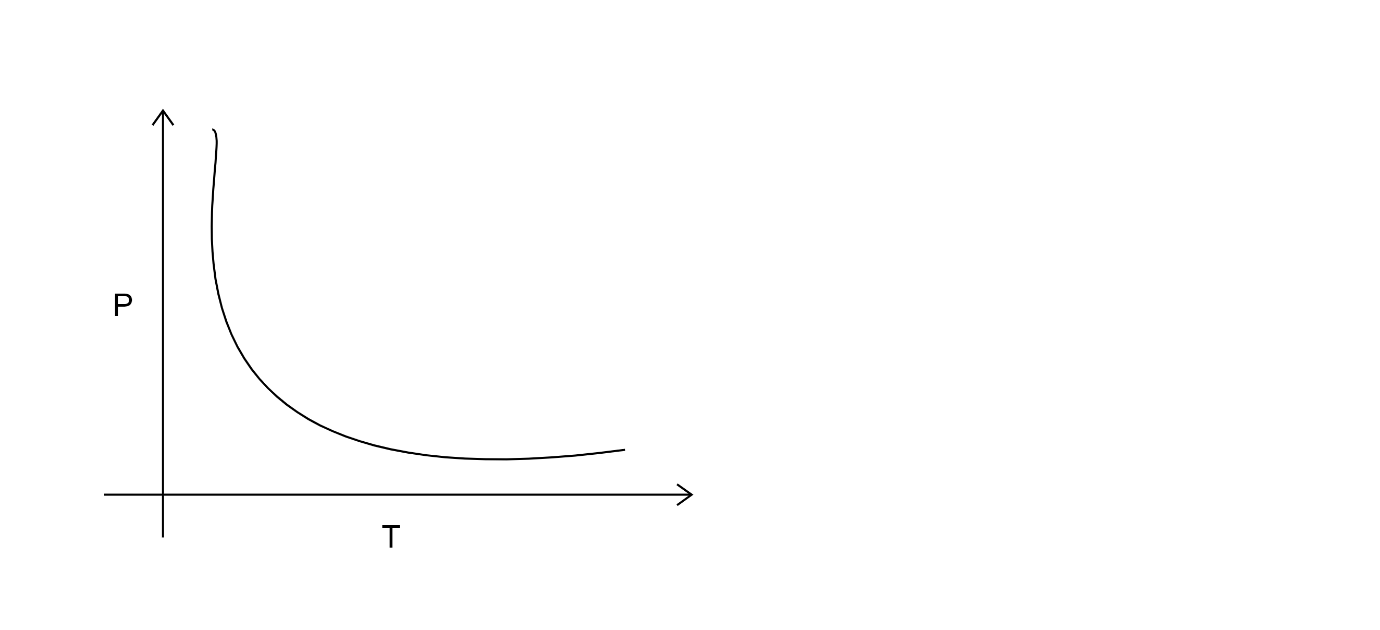 C.
C.
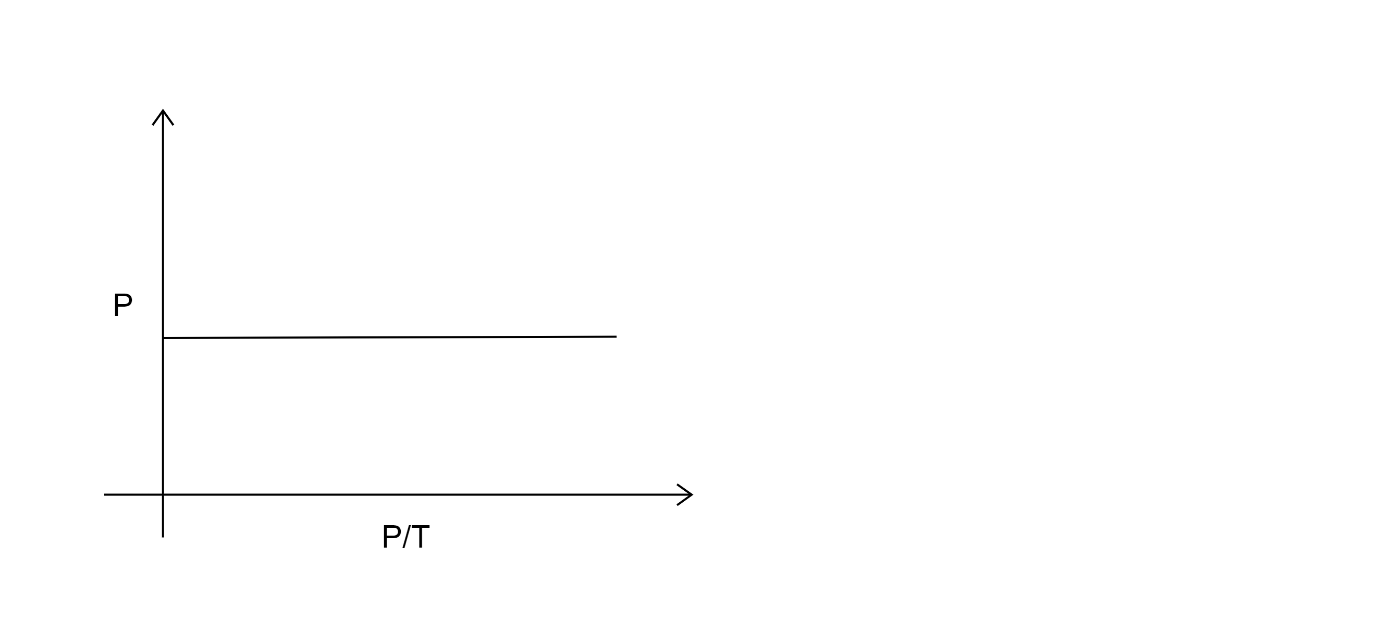 D.
D.
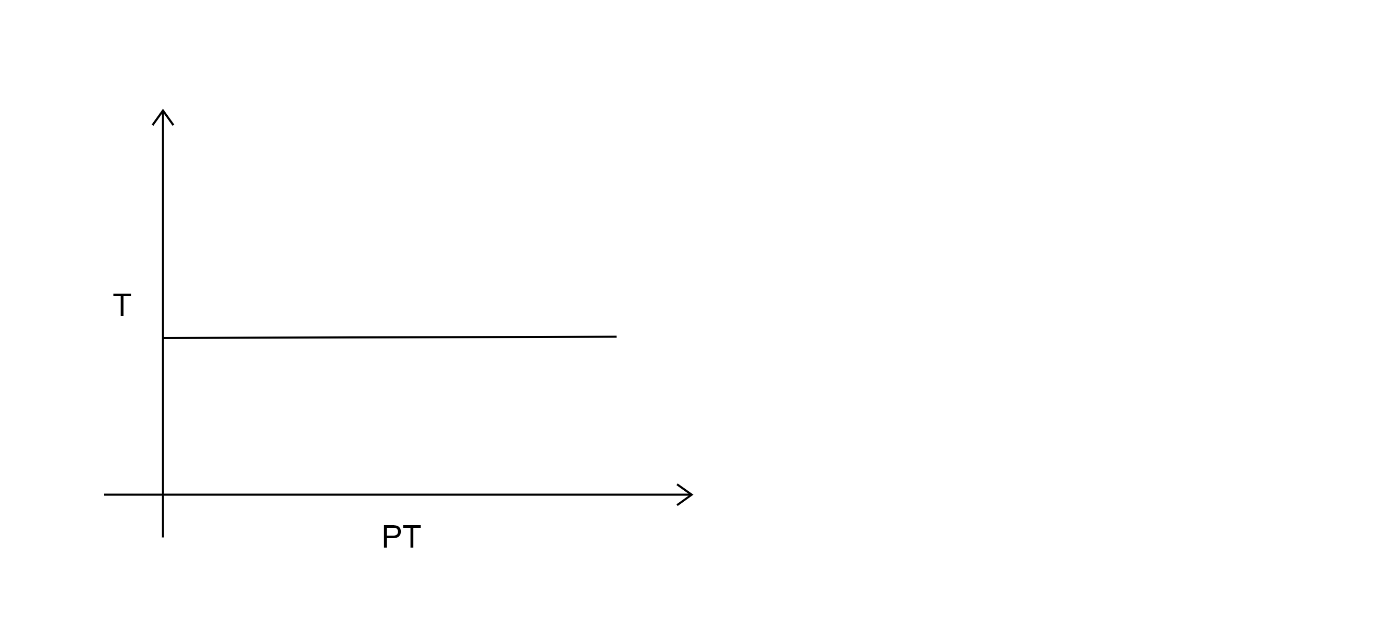




Answer
513k+ views
Hint: Ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas follows ideal gas law and is easy to analyse under statistical mechanisms.
Complete answer: Ideal gas law is the combination of Boyle’s law, Charles’s law, Avogardo’s law and Gay Lussac’s law.
Boyle’s law state that the absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system.
\[{P_1}{V_1} = {P_2}{V_2}\]
Charles’s law states that the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature, if the pressure remains constant.
\[\dfrac{{{V_1}}}{{{T_1}}} = \dfrac{{{V_2}}}{{{T_2}}}\]
Avogardo’s law states that under same conditions of temperature and pressure, equal volumes of different gases contain equal number of molecules. It is given by the formula-
\[\dfrac{{{V_1}}}{{{n_1}}} = \dfrac{{{V_2}}}{{{n_2}}}\]
Gay Lussac’s law states that the pressure of a given mass of a gas varies with absolute temperature of gas when volume is kept constant. This means that at a constant volume the pressure of the given gas is directly proportional to the temperature. Mathematically it can be written as:
\[\dfrac{P}{T} = k\]
This means that the ration of pressure and temperature is constant .This translates, in terms of graphical representation that the graph having straight line representation will satisfy the law.
Now that we have understood the laws that when comnined forms the ideal gas law let us look at the different graphs
In the first option pressure is decreasing with time so it cannot be the answer.
In option b and d as well the graph does not show direct relation with pressure and temperature.
In the third option the ratio of pressure and temperature remains constant with time, therefore it is our answer that is option C.
Note:
Boyle’s law gives the relation of pressure with volume, Charles’s law gives relation between volume and temperature, Avogadro’s law provides molar mass and volume relation and Gay Lussac’s law gives constant relation of pressure and temperature. These laws when combined together give the ideal gas law.
Complete answer: Ideal gas law is the combination of Boyle’s law, Charles’s law, Avogardo’s law and Gay Lussac’s law.
Boyle’s law state that the absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system.
\[{P_1}{V_1} = {P_2}{V_2}\]
Charles’s law states that the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature, if the pressure remains constant.
\[\dfrac{{{V_1}}}{{{T_1}}} = \dfrac{{{V_2}}}{{{T_2}}}\]
Avogardo’s law states that under same conditions of temperature and pressure, equal volumes of different gases contain equal number of molecules. It is given by the formula-
\[\dfrac{{{V_1}}}{{{n_1}}} = \dfrac{{{V_2}}}{{{n_2}}}\]
Gay Lussac’s law states that the pressure of a given mass of a gas varies with absolute temperature of gas when volume is kept constant. This means that at a constant volume the pressure of the given gas is directly proportional to the temperature. Mathematically it can be written as:
\[\dfrac{P}{T} = k\]
This means that the ration of pressure and temperature is constant .This translates, in terms of graphical representation that the graph having straight line representation will satisfy the law.
Now that we have understood the laws that when comnined forms the ideal gas law let us look at the different graphs
In the first option pressure is decreasing with time so it cannot be the answer.
In option b and d as well the graph does not show direct relation with pressure and temperature.
In the third option the ratio of pressure and temperature remains constant with time, therefore it is our answer that is option C.
Note:
Boyle’s law gives the relation of pressure with volume, Charles’s law gives relation between volume and temperature, Avogadro’s law provides molar mass and volume relation and Gay Lussac’s law gives constant relation of pressure and temperature. These laws when combined together give the ideal gas law.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




