
Which of the following contains three centre and two electron bonds?
(A)- ${\left( {{\text{Be}}{{\text{H}}_{\text{2}}}} \right)_{\text{2}}}$
(B)- ${\text{LiAl}}{{\text{H}}_{\text{4}}}$
(C)- ${\left( {{\text{BeC}}{{\text{l}}_{\text{2}}}} \right)_2}$
(D)- ${\text{L}}{{\text{i}}_{\text{2}}}{{\text{C}}_{\text{2}}}$
Answer
588.6k+ views
Hint: As the name suggests, ‘three centre and two electron bonds’ means we have to choose that compound in which 3 centers is presented as atoms and 2 bonds is present in the given compound.
Complete step by step solution: Three centre and two electron bonds containing compounds are generally electron deficient in nature because 2 electrons are shared in between three atoms.
Among the given options in the question option (A) i.e. ${\left( {{\text{Be}}{{\text{H}}_{\text{2}}}} \right)_{\text{2}}}$is correct, below is given some important points which throw light on the reason:
- Atomic no. of Beryllium (Be) atoms is 4 and its electronic configuration is$1{s^2}2{s^2}$ .
- There are 2 electrons in the valence shell, it means we use these 2 electrons for the formation of bonds.
- Among these 2 electrons, one electron forms a covalent bond with one hydrogen atom and another electron is used to form a banana bond with another hydrogen atom.
- In the given compound${\left( {{\text{Be}}{{\text{H}}_{\text{2}}}} \right)_{\text{2}}}$, total no. of Be atom is 2 and total no. of hydrogen atom is 4; among which 2 hydrogen forms covalent bond & another 2 takes part in the formation of banana bond.
- One Banana bond is formed by the 2 electrons between the 3 atoms but there is no uniform distribution of electrons among all 3 atoms, it is more towards the 2 atoms only.
- The structure of ${\left( {{\text{Be}}{{\text{H}}_{\text{2}}}} \right)_{\text{2}}}$is shown as:
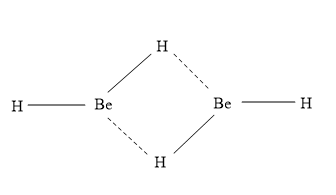
- Here Be has $s{p^2}$ hybridization.
- Be-H-Be is a 3 centre between them 2 electron bonds are present, which is known as banana bond.
So, option A is the correct answer.
Note: In this question it is mentioned that between 3 centre, 2 electron bonds is present so never confuse with the Be-H bond because in this bond 2 centre is only present. And as Be forms 3 bonds but among these 3 bonds, electrons are not present in that bond which is shown by the dash line.
Complete step by step solution: Three centre and two electron bonds containing compounds are generally electron deficient in nature because 2 electrons are shared in between three atoms.
Among the given options in the question option (A) i.e. ${\left( {{\text{Be}}{{\text{H}}_{\text{2}}}} \right)_{\text{2}}}$is correct, below is given some important points which throw light on the reason:
- Atomic no. of Beryllium (Be) atoms is 4 and its electronic configuration is$1{s^2}2{s^2}$ .
- There are 2 electrons in the valence shell, it means we use these 2 electrons for the formation of bonds.
- Among these 2 electrons, one electron forms a covalent bond with one hydrogen atom and another electron is used to form a banana bond with another hydrogen atom.
- In the given compound${\left( {{\text{Be}}{{\text{H}}_{\text{2}}}} \right)_{\text{2}}}$, total no. of Be atom is 2 and total no. of hydrogen atom is 4; among which 2 hydrogen forms covalent bond & another 2 takes part in the formation of banana bond.
- One Banana bond is formed by the 2 electrons between the 3 atoms but there is no uniform distribution of electrons among all 3 atoms, it is more towards the 2 atoms only.
- The structure of ${\left( {{\text{Be}}{{\text{H}}_{\text{2}}}} \right)_{\text{2}}}$is shown as:

- Here Be has $s{p^2}$ hybridization.
- Be-H-Be is a 3 centre between them 2 electron bonds are present, which is known as banana bond.
So, option A is the correct answer.
Note: In this question it is mentioned that between 3 centre, 2 electron bonds is present so never confuse with the Be-H bond because in this bond 2 centre is only present. And as Be forms 3 bonds but among these 3 bonds, electrons are not present in that bond which is shown by the dash line.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




