
Which of the following contain $P - P$ bond?
(A) \[{P_2}O_7^{4 - }\]
(B) \[{P_2}O_6^{3 - }\]
(C) \[{P_2}O_9^{3 - }\]
(D) \[{P_2}O_6^{4 - }\]
Answer
588.9k+ views
Hint: P-P bond means two phosphorus atoms are joined with each other and form a bridge between the molecules. P-P bond is a covalent bond, formed by the sharing of electrons by different atoms present in a molecule.
Complete step by step answer:
The prefix 'hypo-' means beneath or less than. Hypophosphoric acid has an oxidation state as \[ + 4\] that is in between phosphoric acid and phosphorus acid. Thus, "hypophosphoric" refers to less than phosphoric but above the phosphorous acid.
Among the given options, hypophosphoric acid i.e. \[{H_4}{P_2}O_6^{4 - }\] is a mineral acid. It has a \[ + 4\] oxidation state. The two phosphorus atoms of the molecule form a covalent P-P bond as shown in the figure.
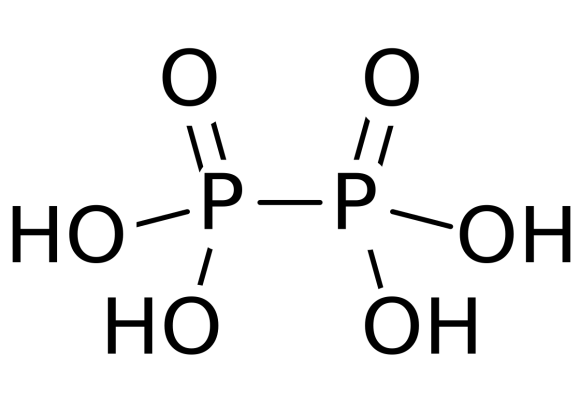
The structure of hypophosphorous acid is containing two P-O bonds with \[151{\text{ }}pm\] length, \[P - OH\] bond with \[151{\text{ }}pm\] length and P-P bond of \[219{\text{ }}pm\] length. Hypophosphoric acid also has oxonium ions with formula ${[{H_3}{O^ + }]_2}{[{H_2}{P_2}{O_6}]^{2 - }}$ . The hypophosphoric acid is isostructural with the diammonium salt which containing ${[HOP{O_2}P{O_2}OH]^{2 - }}$ anion with a P−P bond length of \[219{\text{ }}pm.\]
Dihydrate ${H_4}{O_2}{P_6}.2{H_2}O$ is the solid state of hypophosphoric acid, formed by the reaction of red phosphorus and sodium chlorite at room temperature.
$2P + 2NaCl{O_2} + 2{H_2}O \to N{a_2}{H_2}{P_2}{O_6} + 2HCl$
Hence, the correct option is (A) \[{P_2}O_7^{4 - }\].
Additional information:
Hypophosphoric acid is used as a bleaching agent, reducing agent, stimulant in pharmaceutical agents and as a wetting agent. Hypophosphoric acid is triprotic and have dissociation constants \[p{K_{a1}}\; = {\text{ }}2.2,{\text{ }}p{K_{a2}}\; = {\text{ }}2.8,{\text{ }}p{K_{a3}}\; = {\text{ }}7.3{\text{ }}and{\text{ }}p{K_{a4}}\; = {\text{ }}10.0\].
Note:
In air the hypophosphites oxidise and form pyrophosphates containing the \[{P_2}O_7^{4 - }\] ion where P has an oxidation state of \[ + 5\]. They are stable to alkali hydroxides. It converts to the orthophosphate when fused with sodium hydroxide.
Complete step by step answer:
The prefix 'hypo-' means beneath or less than. Hypophosphoric acid has an oxidation state as \[ + 4\] that is in between phosphoric acid and phosphorus acid. Thus, "hypophosphoric" refers to less than phosphoric but above the phosphorous acid.
Among the given options, hypophosphoric acid i.e. \[{H_4}{P_2}O_6^{4 - }\] is a mineral acid. It has a \[ + 4\] oxidation state. The two phosphorus atoms of the molecule form a covalent P-P bond as shown in the figure.

The structure of hypophosphorous acid is containing two P-O bonds with \[151{\text{ }}pm\] length, \[P - OH\] bond with \[151{\text{ }}pm\] length and P-P bond of \[219{\text{ }}pm\] length. Hypophosphoric acid also has oxonium ions with formula ${[{H_3}{O^ + }]_2}{[{H_2}{P_2}{O_6}]^{2 - }}$ . The hypophosphoric acid is isostructural with the diammonium salt which containing ${[HOP{O_2}P{O_2}OH]^{2 - }}$ anion with a P−P bond length of \[219{\text{ }}pm.\]
Dihydrate ${H_4}{O_2}{P_6}.2{H_2}O$ is the solid state of hypophosphoric acid, formed by the reaction of red phosphorus and sodium chlorite at room temperature.
$2P + 2NaCl{O_2} + 2{H_2}O \to N{a_2}{H_2}{P_2}{O_6} + 2HCl$
Hence, the correct option is (A) \[{P_2}O_7^{4 - }\].
Additional information:
Hypophosphoric acid is used as a bleaching agent, reducing agent, stimulant in pharmaceutical agents and as a wetting agent. Hypophosphoric acid is triprotic and have dissociation constants \[p{K_{a1}}\; = {\text{ }}2.2,{\text{ }}p{K_{a2}}\; = {\text{ }}2.8,{\text{ }}p{K_{a3}}\; = {\text{ }}7.3{\text{ }}and{\text{ }}p{K_{a4}}\; = {\text{ }}10.0\].
Note:
In air the hypophosphites oxidise and form pyrophosphates containing the \[{P_2}O_7^{4 - }\] ion where P has an oxidation state of \[ + 5\]. They are stable to alkali hydroxides. It converts to the orthophosphate when fused with sodium hydroxide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




