
Which of the following compounds will not be soluble in sodium bicarbonate?
A) $ {\text{2,4,6 - Trinitrophenol}} $
B) Benzoic acid
C) o-Nitrophenol
D) Benzene sulphonic acid
Answer
489.6k+ views
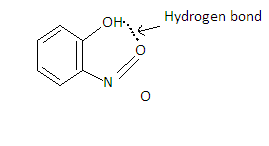
Hint: o-Nitro phenol has intramolecular hydrogen bonding which makes it difficult to release the $ {{\text{H}}^ + } $ ion. So it is a weak acid which will not show any reaction when reacted with sodium bicarbonate. Hence it is not soluble in sodium bicarbonate.
Complete answer:
The given compounds will be soluble in sodium bicarbonate only if they have higher acidity. This means that they can easily give $ {{\text{H}}^ + } $ ion and form sodium salts of acid. Here, $ {\text{2,4,6 - Trinitrophenol}} $ has higher acidity than o-Nitrophenol as it has 3 $ {\text{ - N}}{{\text{O}}_{\text{2}}} $ groups which is electron withdrawing group so it increases the acidic strength of phenol. So, it will be soluble in sodium bicarbonate. The reaction will be-
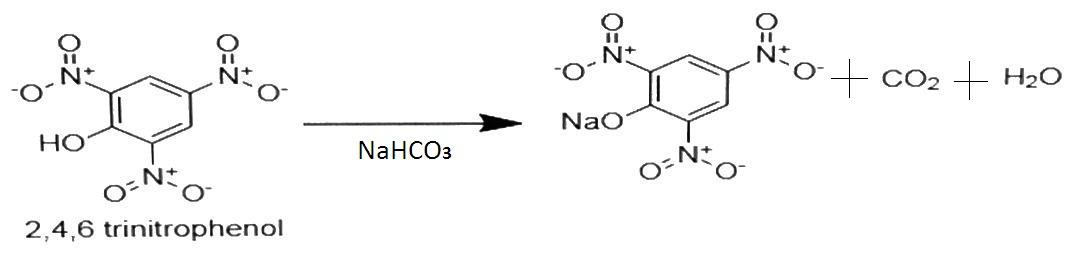
The product formed is the sodium salt of acid. Benzoic acid and Benzene sulphonic acid are strong acids as they give $ {{\text{H}}^ + } $ ion easily. So they are soluble in sodium bicarbonate. The reaction is as follows-
$ \Rightarrow {{\text{C}}_6}{{\text{H}}_5}{\text{COOH + NaHC}}{{\text{O}}_3} \to {\text{Na}}{{\text{C}}_6}{{\text{H}}_5}{\text{COO + }}{{\text{H}}_2}{\text{O + C}}{{\text{O}}_2} \\
{{\text{C}}_6}{{\text{H}}_5}{\text{S}}{{\text{O}}_2}{\text{OH + NaHC}}{{\text{O}}_3} \to {{\text{C}}_6}{{\text{H}}_5}{\text{S}}{{\text{O}}_2}{\text{ONa + }}{{\text{H}}_2}{\text{O + C}}{{\text{O}}_2} \\ $
Hence the answer is ‘C’.
Note:
Hydrogen Bonding is the bonding between the hydrogen atom of one molecule with the electronegative atom of an adjacent molecule. It is represented by (-). Hydrogen bonding is of two types-
(i)Intermolecular hydrogen bonding- Hydrogen bond formed between 2 molecules
(ii)Intermolecular hydrogen bonding-Hydrogen bond formed within the molecule.
In o-Nitrophenol, Intermolecular hydrogen bonding is present.
Complete answer:
The given compounds will be soluble in sodium bicarbonate only if they have higher acidity. This means that they can easily give $ {{\text{H}}^ + } $ ion and form sodium salts of acid. Here, $ {\text{2,4,6 - Trinitrophenol}} $ has higher acidity than o-Nitrophenol as it has 3 $ {\text{ - N}}{{\text{O}}_{\text{2}}} $ groups which is electron withdrawing group so it increases the acidic strength of phenol. So, it will be soluble in sodium bicarbonate. The reaction will be-

The product formed is the sodium salt of acid. Benzoic acid and Benzene sulphonic acid are strong acids as they give $ {{\text{H}}^ + } $ ion easily. So they are soluble in sodium bicarbonate. The reaction is as follows-
$ \Rightarrow {{\text{C}}_6}{{\text{H}}_5}{\text{COOH + NaHC}}{{\text{O}}_3} \to {\text{Na}}{{\text{C}}_6}{{\text{H}}_5}{\text{COO + }}{{\text{H}}_2}{\text{O + C}}{{\text{O}}_2} \\
{{\text{C}}_6}{{\text{H}}_5}{\text{S}}{{\text{O}}_2}{\text{OH + NaHC}}{{\text{O}}_3} \to {{\text{C}}_6}{{\text{H}}_5}{\text{S}}{{\text{O}}_2}{\text{ONa + }}{{\text{H}}_2}{\text{O + C}}{{\text{O}}_2} \\ $
Hence the answer is ‘C’.
Note:
Hydrogen Bonding is the bonding between the hydrogen atom of one molecule with the electronegative atom of an adjacent molecule. It is represented by (-). Hydrogen bonding is of two types-
(i)Intermolecular hydrogen bonding- Hydrogen bond formed between 2 molecules
(ii)Intermolecular hydrogen bonding-Hydrogen bond formed within the molecule.
In o-Nitrophenol, Intermolecular hydrogen bonding is present.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




