
Which of the following compounds undergoes replacement of –Cl by –OH by merely warming the compound with aqueous NaOH?
(a)-
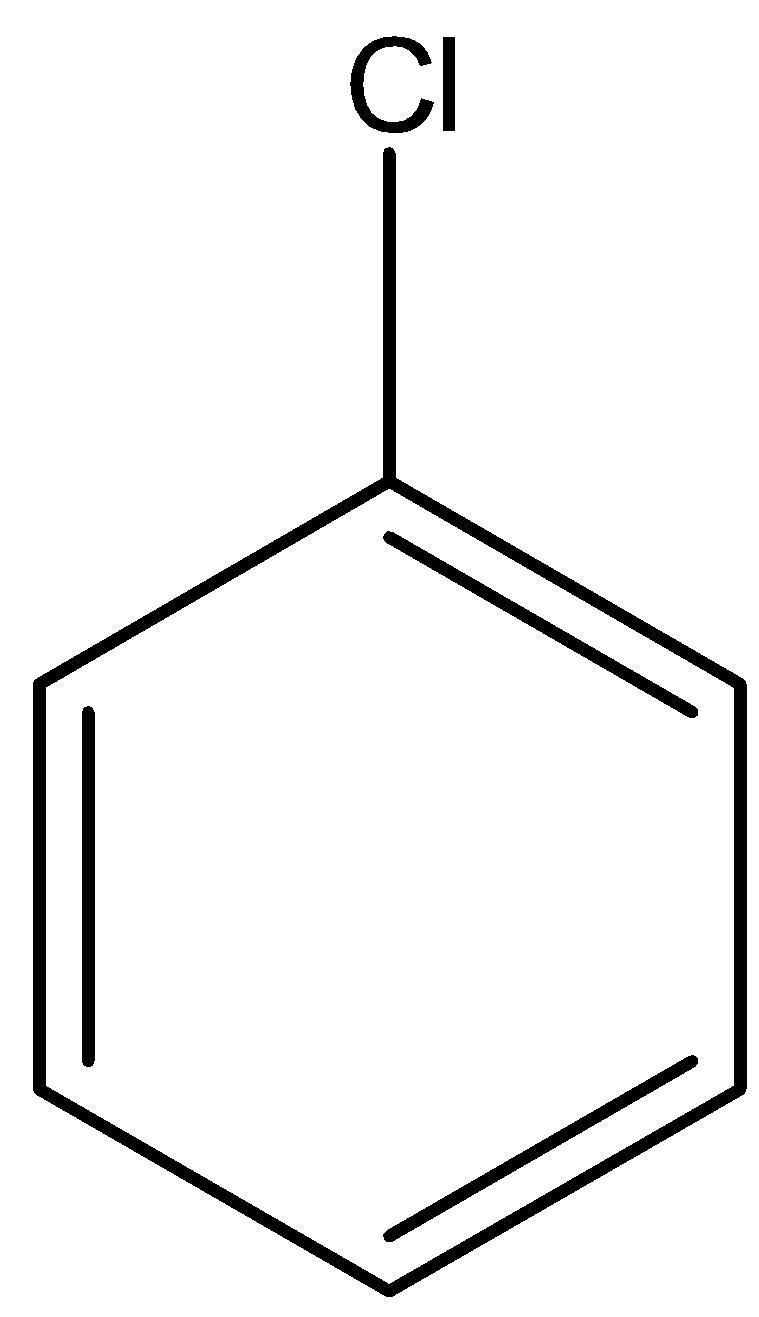
(b)-
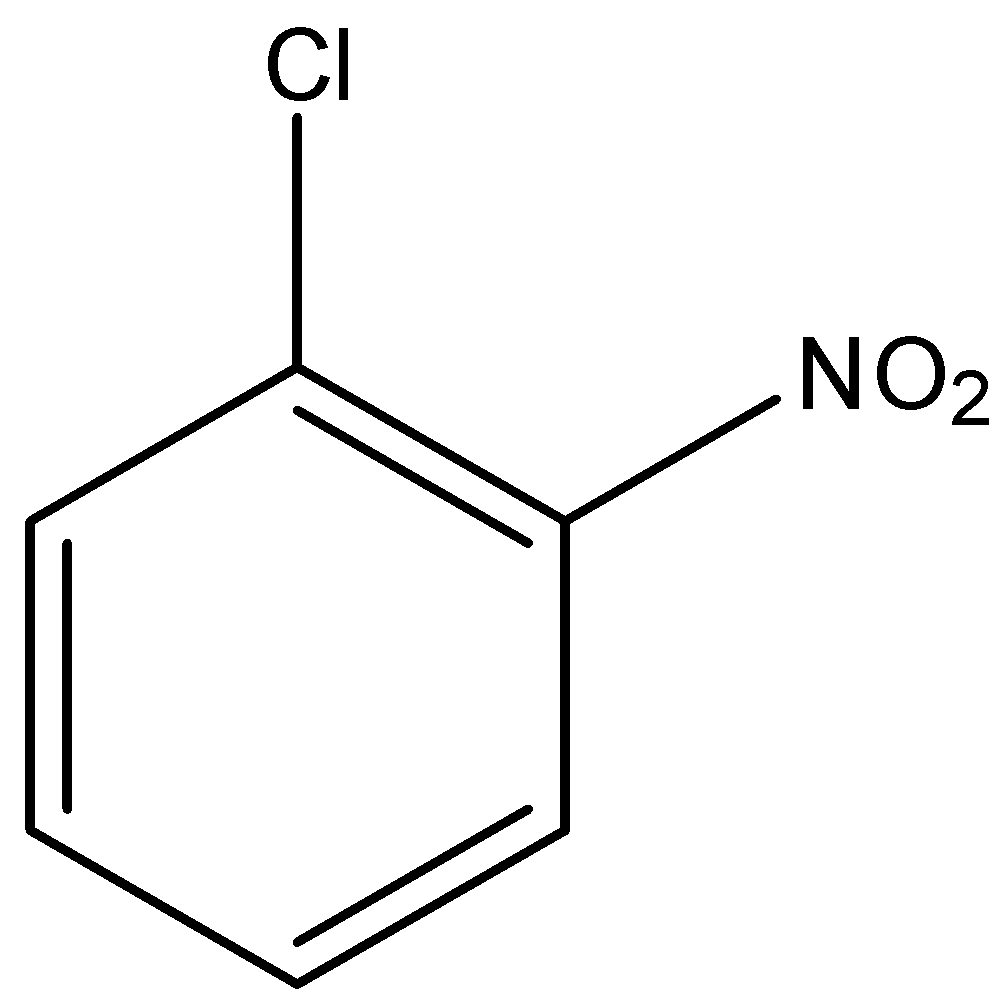
(c)-
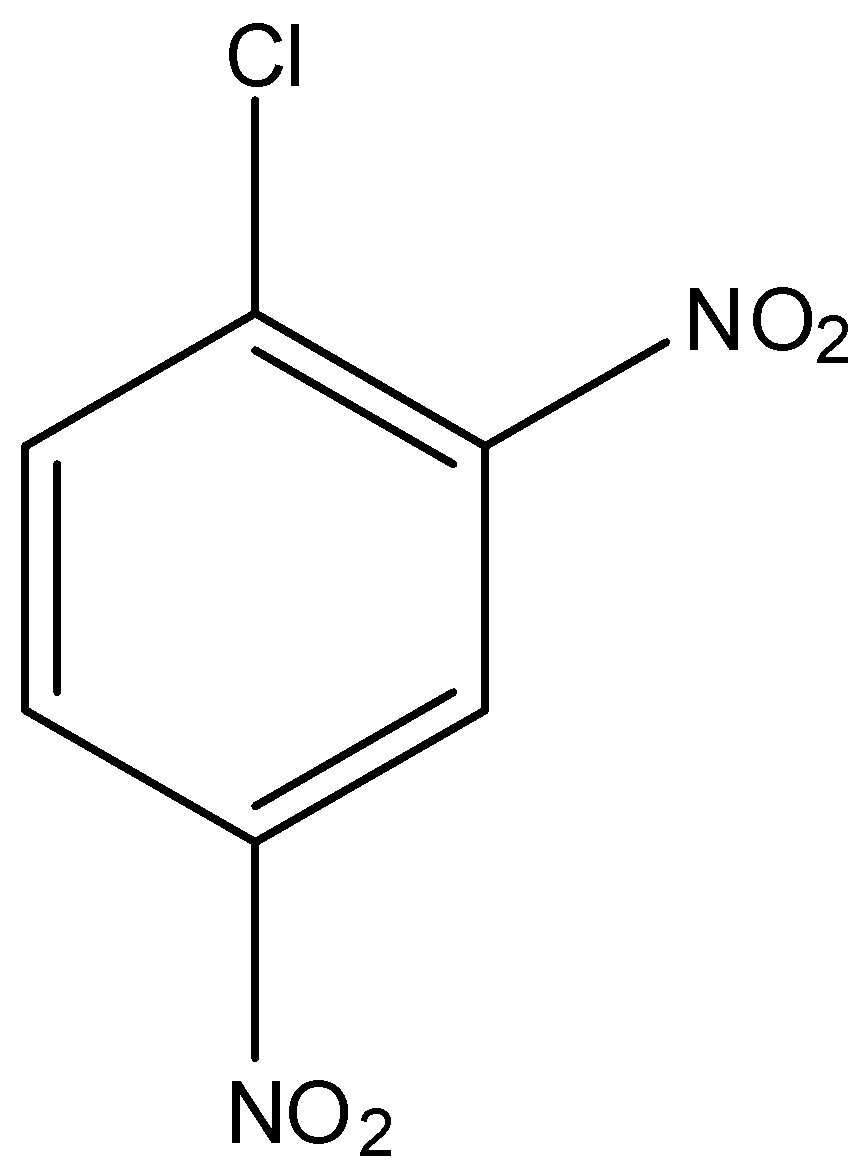
(d)-
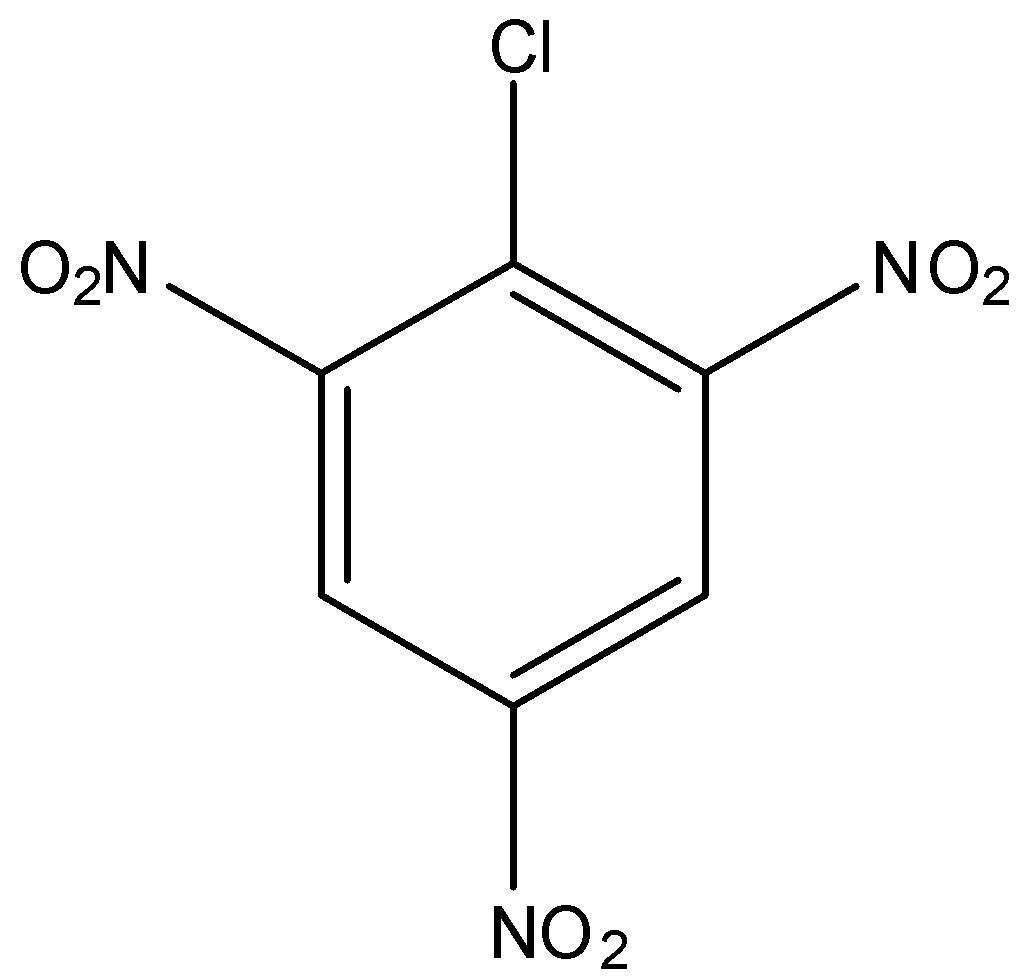




Answer
531.6k+ views
Hint: Both the –Cl and –OH are nucleophiles, so when –Cl is exchanged with –OH ion then it is known as Aromatic Nucleophilic substitution reaction. As the electron-withdrawing group increases on the benzene ring then the replacement will be easy.
Complete answer:
Both the –Cl (Chloride ion) and –OH (hydroxyl ion) are nucleophiles, so when –Cl is exchanged with –OH ion then it is known as an Aromatic Nucleophilic substitution reaction. Nucleophilic substitution reaction is not easy in the aromatic compounds.
Hence, there must be some electron-withdrawing group on the benzene ring, so that there can be substitution of the group because the electron-withdrawing increases the activity of the benzene ring and increases the reactivity. As the number of an electron-withdrawing group increases on the benzene ring then the replacement will be easy.
In option (a) there is no electron-withdrawing group, in option (b) there is one nitro group present at the ortho position, in option (c) there is two nitro group present at ortho and para position, and in option (d) there is three nitro group present at two orthos and one para position. As we know that the nitro group is a strong electron-withdrawing group, therefore, the compound with three nitro groups will have the highest reactivity towards the Nucleophilic substitution reaction.
Hence, the correct answer is an option (d)-
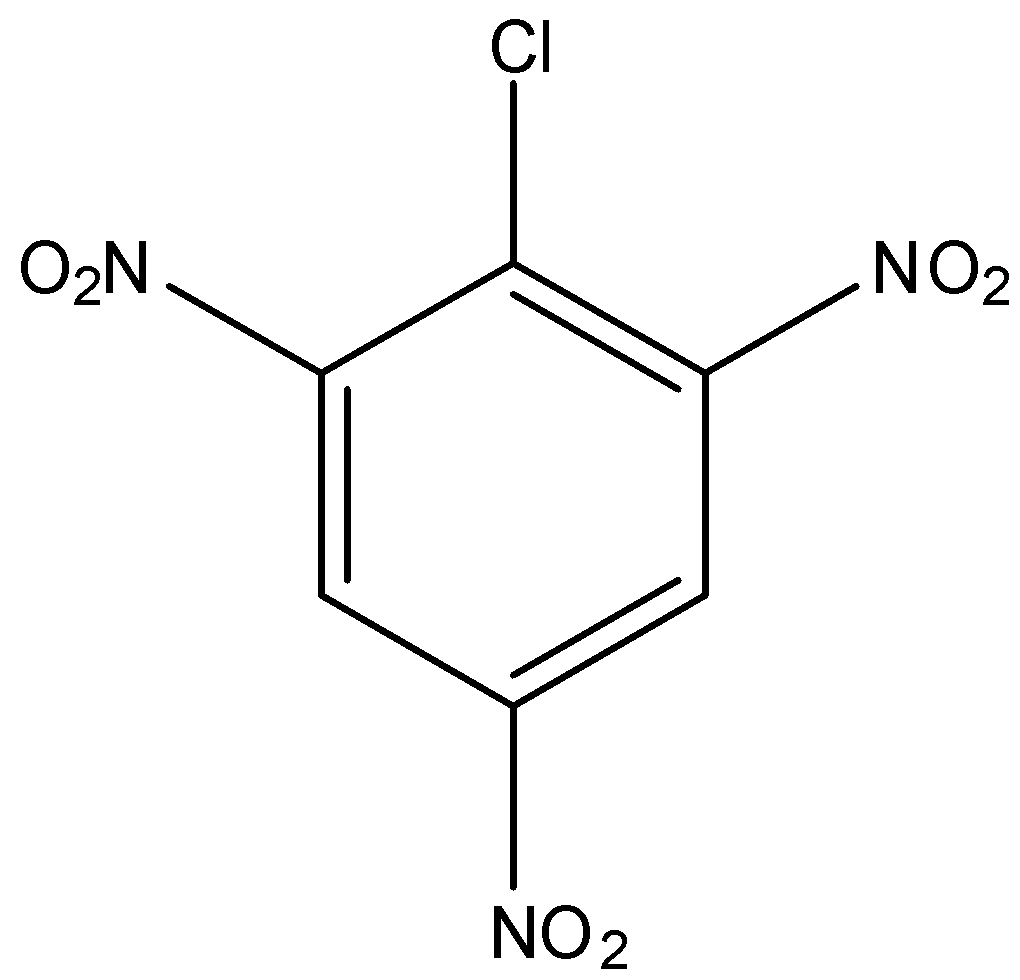
Note:
As compared to the nucleophilic substitution reaction, the aromatic compounds undergo Electrophilic substitution reactions easily. If the number of electron-donating groups increases on the benzene ring then the reactivity will decrease.
Complete answer:
Both the –Cl (Chloride ion) and –OH (hydroxyl ion) are nucleophiles, so when –Cl is exchanged with –OH ion then it is known as an Aromatic Nucleophilic substitution reaction. Nucleophilic substitution reaction is not easy in the aromatic compounds.
Hence, there must be some electron-withdrawing group on the benzene ring, so that there can be substitution of the group because the electron-withdrawing increases the activity of the benzene ring and increases the reactivity. As the number of an electron-withdrawing group increases on the benzene ring then the replacement will be easy.
In option (a) there is no electron-withdrawing group, in option (b) there is one nitro group present at the ortho position, in option (c) there is two nitro group present at ortho and para position, and in option (d) there is three nitro group present at two orthos and one para position. As we know that the nitro group is a strong electron-withdrawing group, therefore, the compound with three nitro groups will have the highest reactivity towards the Nucleophilic substitution reaction.
Hence, the correct answer is an option (d)-

Note:
As compared to the nucleophilic substitution reaction, the aromatic compounds undergo Electrophilic substitution reactions easily. If the number of electron-donating groups increases on the benzene ring then the reactivity will decrease.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




