
Which of the following compounds on boiling with alkaline potassium permanganate and subsequent acidification will not give benzoic acid?
A. Benzyl alcohol
B. Acetophenone
C. Anisole
D. Toluene
Answer
373.5k+ views
Hint: Alkaline potassium permanganate (KMnO4) solution is named Bayer's reagent.
It is a powerful oxidising agent which oxidises benzyl alcohol, acetophenone, and toluene to benzoic acid.
Complete Step by Step Answer:
Alkaline potassium permanganate is a strong oxidising agent which oxidises organic compounds undergoing reduction itself. Benzoic acid is a white solid with the chemical formula \[{C_6}{H_5}COOH\].
It is the simplest aromatic carboxylic acid. Out of the given options, we have to find out the compound which on boiling with alkaline potassium permanganate and subsequent acidification will not give benzoic acid.
A. Benzyl alcohol
Benzyl alcohol is an aromatic alcohol with the chemical formula \[{C_6}{H_5}C{H_2}OH\]. It is a colourless liquid with a nice aromatic odour. Alcohols undergo oxidation with the formation of a carbon-oxygen double bond with cleavage of O-H and C-H bonds. Benzyl alcohol is a primary alcohol that in the presence of KMnO4 gets oxidised to benzaldehyde and then to benzoic acid.
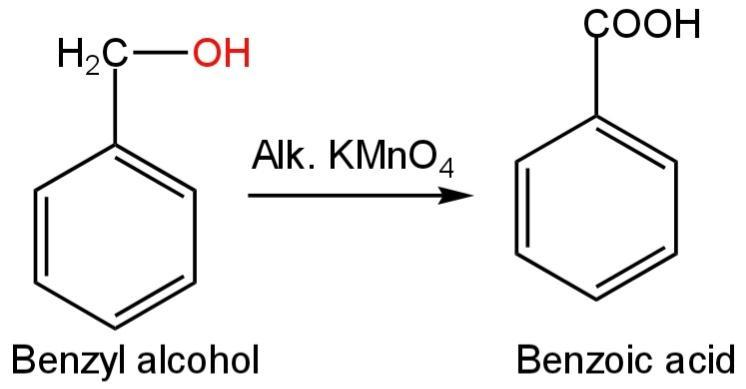
Image: Oxidation of benzyl alcohol
So, benzyl alcohol on boiling with alkaline potassium permanganate will give benzoic acid.
So, A is incorrect.
B. Acetophenone
Acetophenone is the organic compound with the formula. It is a very simple aromatic ketone.
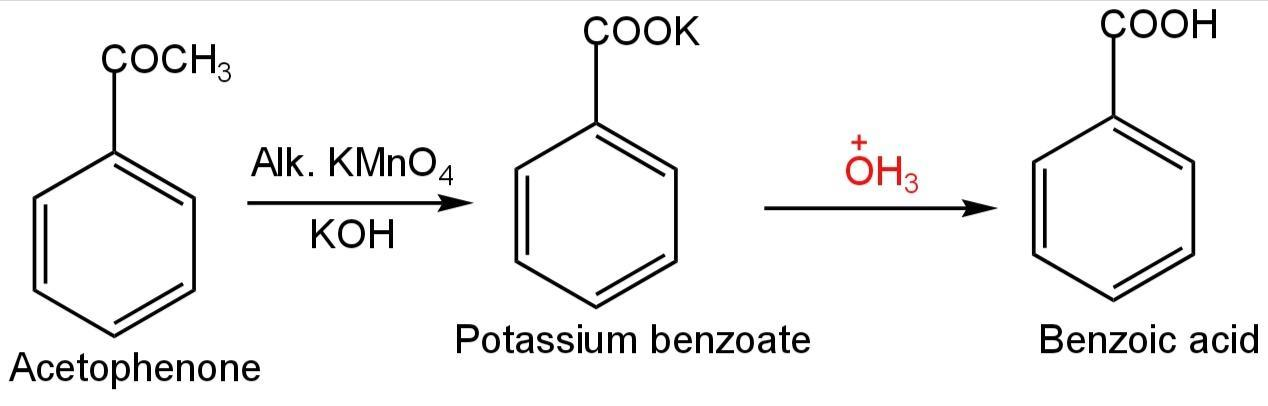
Image: Oxidation of acetophenone
This compound on reaction with\[KMn{O_4}\]and KOH forms potassium benzoate which on acidification forms benzoic acid.
So, B is incorrect.
C. Anisole
Anisole is a chemical compound with the chemical formula \[C{H_3}O{C_6}{H_5}\]. It is also known as methoxybenzene. Its structure is as follows:
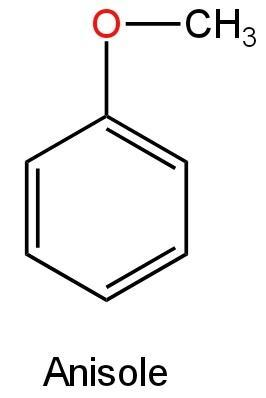
Image: Anisole
This is an ether compound that will not undergo oxidation with potassium permanganate to form benzoic acid. So, C is correct.
D. Toluene
Toluene is a substituted aromatic hydrocarbon. It is a mono-substituted benzene derivative carrying a methyl group attached to a phenyl group. Its IUPAC name is methylbenzene. This compound on oxidation with alkaline potassium permanganate and will give benzoic acid.
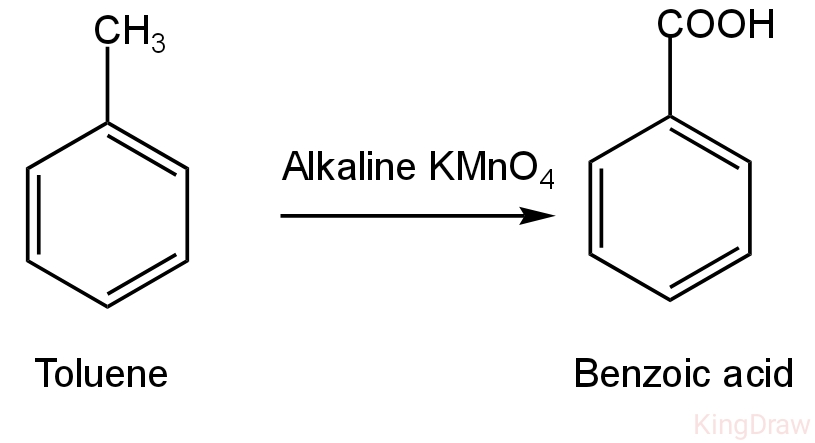
Image: Oxidation of toluene
So, anisole will not undergo oxidation with potassium permanganate to form benzoic acid.
So, option C is correct.
Note: An oxidising agent undergoes reduction to oxidise others. It gains the electrons to get reduced and contribute its electrons to the compound which gets oxidised. Potassium permanganate has the anion \[MnO_4^ - \], and the oxidation state of manganese in potassium permanganate is +7, which can reduce to +6, +5, +4, +3, or +2 oxidation states.
It is a powerful oxidising agent which oxidises benzyl alcohol, acetophenone, and toluene to benzoic acid.
Complete Step by Step Answer:
Alkaline potassium permanganate is a strong oxidising agent which oxidises organic compounds undergoing reduction itself. Benzoic acid is a white solid with the chemical formula \[{C_6}{H_5}COOH\].
It is the simplest aromatic carboxylic acid. Out of the given options, we have to find out the compound which on boiling with alkaline potassium permanganate and subsequent acidification will not give benzoic acid.
A. Benzyl alcohol
Benzyl alcohol is an aromatic alcohol with the chemical formula \[{C_6}{H_5}C{H_2}OH\]. It is a colourless liquid with a nice aromatic odour. Alcohols undergo oxidation with the formation of a carbon-oxygen double bond with cleavage of O-H and C-H bonds. Benzyl alcohol is a primary alcohol that in the presence of KMnO4 gets oxidised to benzaldehyde and then to benzoic acid.

Image: Oxidation of benzyl alcohol
So, benzyl alcohol on boiling with alkaline potassium permanganate will give benzoic acid.
So, A is incorrect.
B. Acetophenone
Acetophenone is the organic compound with the formula. It is a very simple aromatic ketone.

Image: Oxidation of acetophenone
This compound on reaction with\[KMn{O_4}\]and KOH forms potassium benzoate which on acidification forms benzoic acid.
So, B is incorrect.
C. Anisole
Anisole is a chemical compound with the chemical formula \[C{H_3}O{C_6}{H_5}\]. It is also known as methoxybenzene. Its structure is as follows:

Image: Anisole
This is an ether compound that will not undergo oxidation with potassium permanganate to form benzoic acid. So, C is correct.
D. Toluene
Toluene is a substituted aromatic hydrocarbon. It is a mono-substituted benzene derivative carrying a methyl group attached to a phenyl group. Its IUPAC name is methylbenzene. This compound on oxidation with alkaline potassium permanganate and will give benzoic acid.

Image: Oxidation of toluene
So, anisole will not undergo oxidation with potassium permanganate to form benzoic acid.
So, option C is correct.
Note: An oxidising agent undergoes reduction to oxidise others. It gains the electrons to get reduced and contribute its electrons to the compound which gets oxidised. Potassium permanganate has the anion \[MnO_4^ - \], and the oxidation state of manganese in potassium permanganate is +7, which can reduce to +6, +5, +4, +3, or +2 oxidation states.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

What is the median of the first 10 natural numbers class 10 maths CBSE




