
Which of the following compounds is hemiacetal?
A)
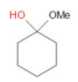
B)
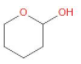
C)
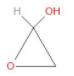
D) All of these



Answer
548.4k+ views
Hint:
To answer this question, you must recall the characteristics of a hemiacetal group. A hemiacetal is a compound which contains a carbon atom attached to a hydroxyl group on one side and to ether on the other. Checking all the options for the hemiacetal group, we would get our answer.
Complete step by step solution:
Hemiacetals are obtained as a result of the addition of an alcohol to a ketone. A hemiacetal group can be represented as
$HC\left( {OH} \right)\left( {OR} \right)R'$
In option A, we can observe that the carbon atom has a hydroxyl group at one end and with ether on the other end. Thus, it is a hemiacetal.
In option B, we can observe that the carbon atom is attached to a hydroxyl group at one end and to ether on the other end. Thus, it is a hemiacetal as well.
In option C, we can observe that the carbon atom is attached to a hydroxyl group at one end and toan ether on the other end. Thus, it is a hemiacetal as well.
So, we can conclude by saying that all the given compounds are hemiacetals.
Hence, the correct option is D.
Note:
Acetal group and hemiacetal group are very similar in both their names as well as their structures. Acetal group contains a carbon atom bonded to two ether groups at the same time and is thus very similar to the hemiacetal group in which the carbon atom is attached to an ether group and a hydroxyl group. On reaction with oxidizing agents, they give aldehyde and alcohol.
To answer this question, you must recall the characteristics of a hemiacetal group. A hemiacetal is a compound which contains a carbon atom attached to a hydroxyl group on one side and to ether on the other. Checking all the options for the hemiacetal group, we would get our answer.
Complete step by step solution:
Hemiacetals are obtained as a result of the addition of an alcohol to a ketone. A hemiacetal group can be represented as
$HC\left( {OH} \right)\left( {OR} \right)R'$
In option A, we can observe that the carbon atom has a hydroxyl group at one end and with ether on the other end. Thus, it is a hemiacetal.
In option B, we can observe that the carbon atom is attached to a hydroxyl group at one end and to ether on the other end. Thus, it is a hemiacetal as well.
In option C, we can observe that the carbon atom is attached to a hydroxyl group at one end and toan ether on the other end. Thus, it is a hemiacetal as well.
So, we can conclude by saying that all the given compounds are hemiacetals.
Hence, the correct option is D.
Note:
Acetal group and hemiacetal group are very similar in both their names as well as their structures. Acetal group contains a carbon atom bonded to two ether groups at the same time and is thus very similar to the hemiacetal group in which the carbon atom is attached to an ether group and a hydroxyl group. On reaction with oxidizing agents, they give aldehyde and alcohol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




