
Which of the following compounds has the lowest nitrogen – nitrogen bond length?
${{N}_{2}},{{N}_{2}}{{H}_{2}},{{N}_{2}}{{F}_{2}}$ .
A. ${{N}_{2}}$
B. ${{N}_{2}}{{H}_{2}}$
C. ${{N}_{2}}{{F}_{2}}$
D. All have equal bond length.
Answer
563.1k+ views
Hint: We know that the length of a single bond is high when compared to length of the double bond and triple bonds. The length of the double bond is high when compared to the length of the triple bond.
Complete step by step answer:
- In the question it is asked which molecule contains lowest nitrogen – nitrogen bond length in the given molecules.
- To know about the bond length we should know the structures of the given molecules.
- The structure of the nitrogen molecule (${{N}_{2}}$ ) is as follows.
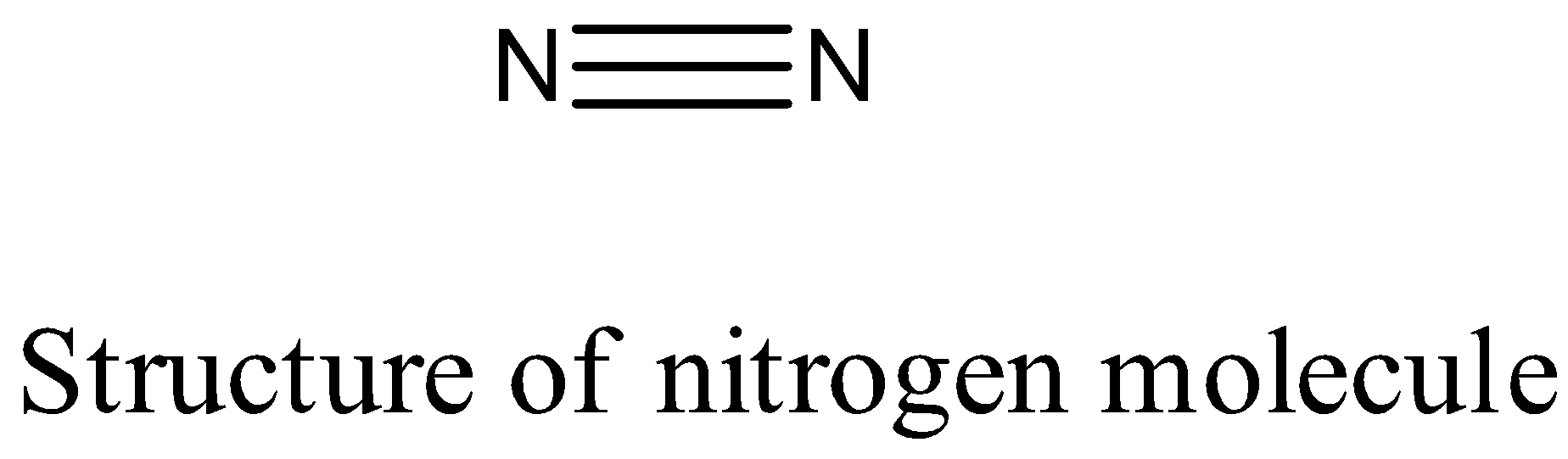
- The nitrogen – nitrogen bond length in nitrogen molecules is 109 pm.
-The structure of the hydrazine ( ${{N}_{2}}{{H}_{2}}$ ) is as follows.
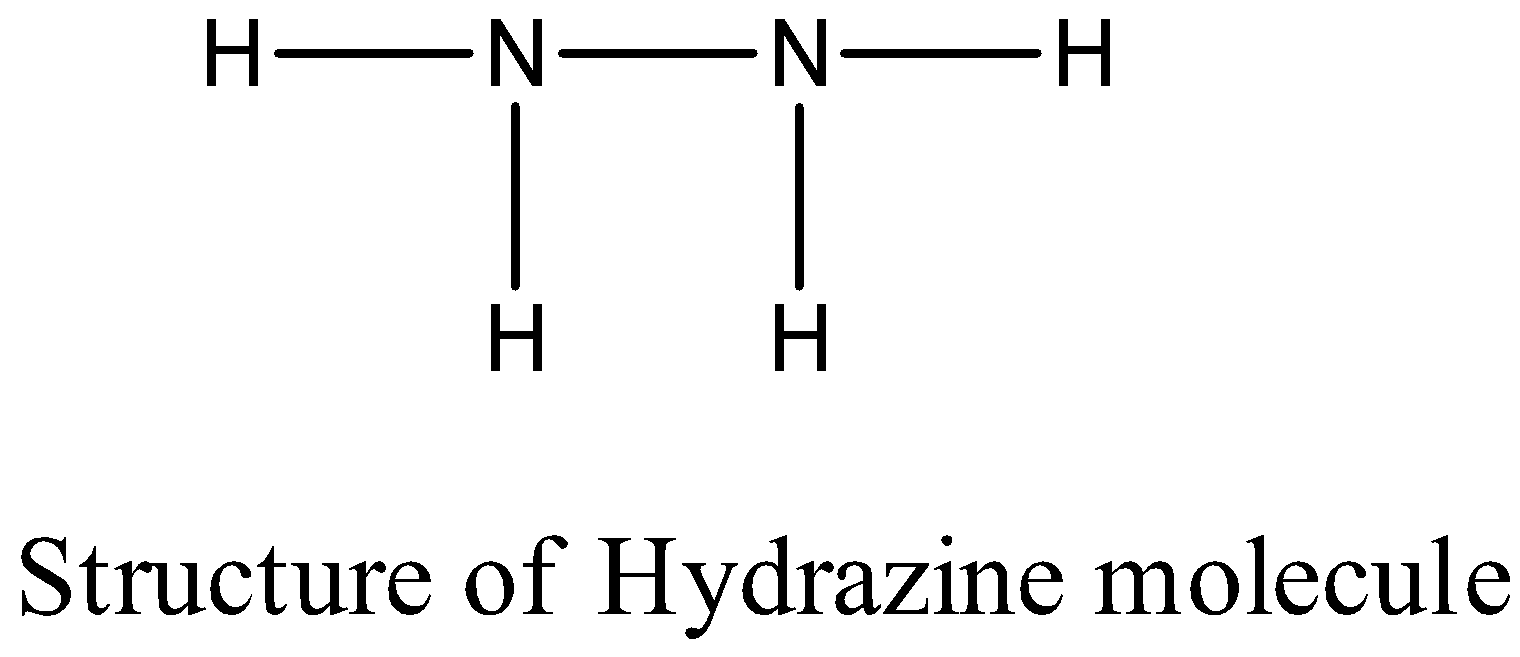
- The nitrogen – nitrogen bond length in the hydrazine molecule is 147 pm.
- Coming to the structure of ${{N}_{2}}{{F}_{2}}$ .
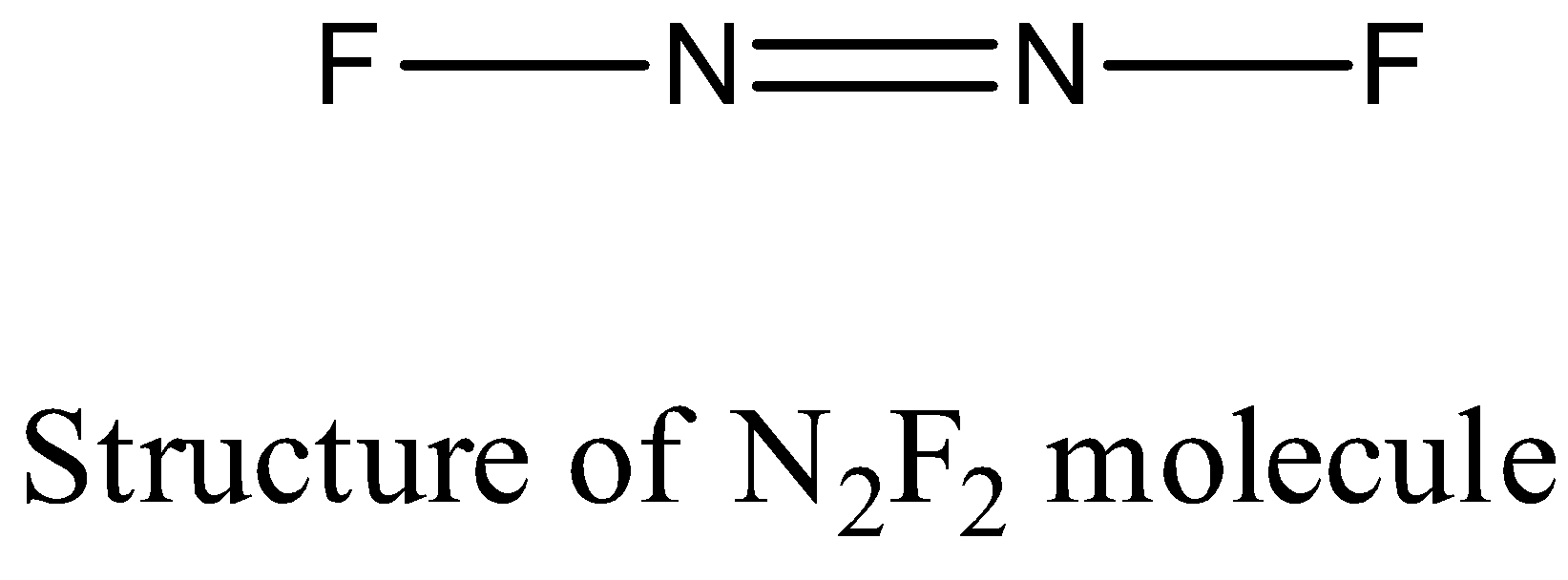
- The nitrogen – nitrogen bond length in ( ${{N}_{2}}{{F}_{2}}$ ) is 122 pm.
- Therefore the nitrogen – nitrogen bond lengths of the given molecules ${{N}_{2}},{{N}_{2}}{{H}_{2}},\text{ }and\text{ }{{N}_{2}}{{F}_{2}}$ are 109 pm, 147 pm and 122 pm respectively.
- So, the nitrogen –nitrogen bond length is lowest in nitrogen molecules.
The correct option is option “A” .
Note: We know that the length of the triple bond is less and it is present in the structure of the nitrogen molecule. The strength of the triple bond is high when compared to the strength of the double and single nitrogen- nitrogen bond length.
Complete step by step answer:
- In the question it is asked which molecule contains lowest nitrogen – nitrogen bond length in the given molecules.
- To know about the bond length we should know the structures of the given molecules.
- The structure of the nitrogen molecule (${{N}_{2}}$ ) is as follows.

- The nitrogen – nitrogen bond length in nitrogen molecules is 109 pm.
-The structure of the hydrazine ( ${{N}_{2}}{{H}_{2}}$ ) is as follows.

- The nitrogen – nitrogen bond length in the hydrazine molecule is 147 pm.
- Coming to the structure of ${{N}_{2}}{{F}_{2}}$ .
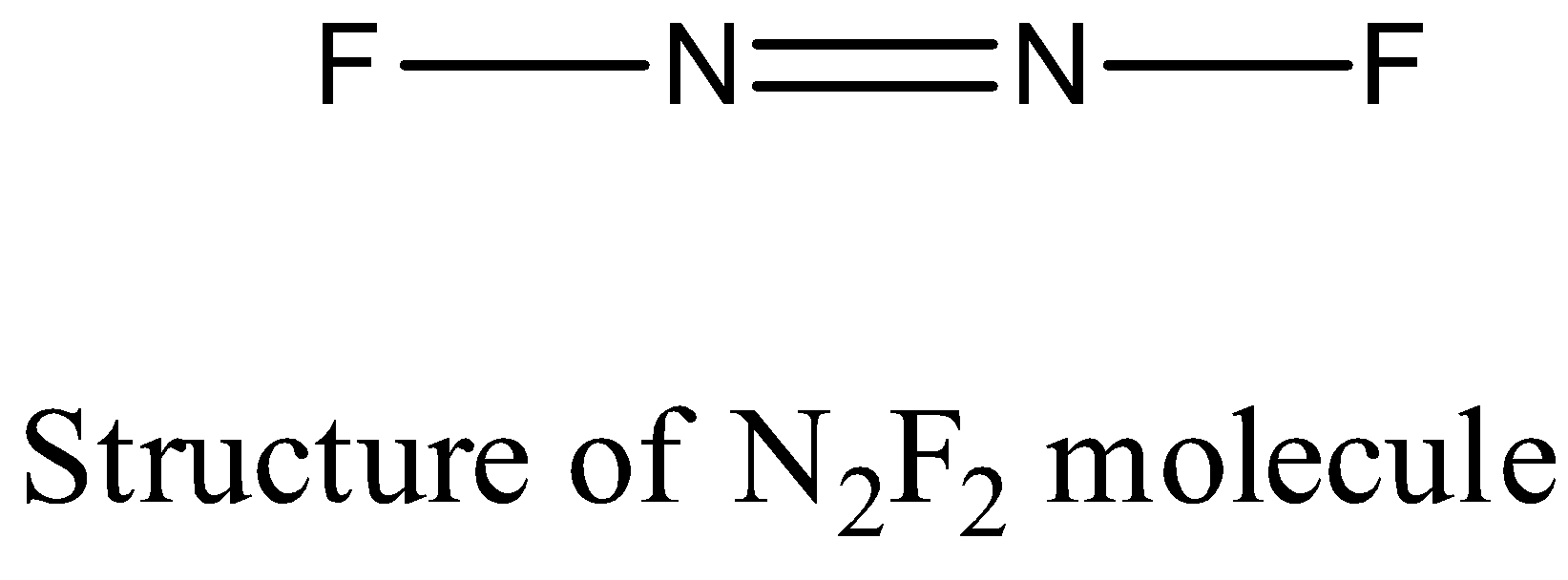
- The nitrogen – nitrogen bond length in ( ${{N}_{2}}{{F}_{2}}$ ) is 122 pm.
- Therefore the nitrogen – nitrogen bond lengths of the given molecules ${{N}_{2}},{{N}_{2}}{{H}_{2}},\text{ }and\text{ }{{N}_{2}}{{F}_{2}}$ are 109 pm, 147 pm and 122 pm respectively.
- So, the nitrogen –nitrogen bond length is lowest in nitrogen molecules.
The correct option is option “A” .
Note: We know that the length of the triple bond is less and it is present in the structure of the nitrogen molecule. The strength of the triple bond is high when compared to the strength of the double and single nitrogen- nitrogen bond length.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




