
Which of the following compounds has a P−P bond?
(A) ${{\left( HP{{O}_{3}} \right)}_{3}}$
(B) ${{H}_{4}}{{P}_{2}}{{O}_{6}}$
(C) ${{H}_{4}}{{P}_{2}}{{O}_{7}}$
(D) ${{H}_{4}}{{P}_{2}}{{O}_{5}}$
Answer
579.6k+ views
Hint: In a P−P bond two phosphorous atoms are bonded with each other and form a bridge between the atoms. The bond between two phosphorus atoms is a covalent bond which is formed by the sharing of electrons by different atoms in a molecule.
Complete step by step solution:
- Among the given options ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ contains the P−P bond. The chemical name of${{H}_{4}}{{P}_{2}}{{O}_{6}}$ is hypophosphoric acid. The acid has an oxidation state of +4. The structure of hypophosphoric acid is given below
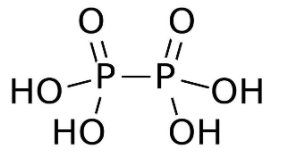
- As we can see in the structure of ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ a single P−P bond is present. Also it contains two P=O bonds and four P−OH bonds .In the structure of ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ the P−O bonds have a bond length of 151 pm and P−P bond has a bond length of 219 pm.
- ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ is a mineral acid and in the solid state it is present as dihydrate which has the molecular formula${{H}_{4}}{{P}_{2}}{{O}_{6}}.2{{H}_{2}}O$. It contains oxonium ions and is formulated as${{\left[ {{H}_{3}}{{O}^{+}} \right]}_{_{2}}}{{\left[ {{H}_{2}}{{P}_{2}}{{O}_{6}} \right]}^{2-}}$.
From the above discussions it’s clear that the compound hypophosphoric acid ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ has a P−P bond in it.
Therefore, the answer is option (B). ${{H}_{4}}{{P}_{2}}{{O}_{6}}$.
Additional information:
The hypophosphoric acid is generally used as reducing agents, wetting agents, as a bleaching agent and as a stimulant in pharmaceutical agents.
Note: Keep in mind that the hypophosphoric acid is a triprotic acid. Also do not confuse Hypophosphorous acid with Hypophosphoric acid. Hypophosphorous acid or phosphinic acid has the molecular formula ${{H}_{3}}P{{O}_{2}}$ whereas Hypophosphoric acid has the molecular formula ${{H}_{4}}{{P}_{2}}{{O}_{6}}$. In addition to this, there are also other oxyacids of phosphorus such as Phosphorus acid, Diphosphoric acid (Pyrophosphoric acid), Peroxophosphoric acid etc.
Complete step by step solution:
- Among the given options ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ contains the P−P bond. The chemical name of${{H}_{4}}{{P}_{2}}{{O}_{6}}$ is hypophosphoric acid. The acid has an oxidation state of +4. The structure of hypophosphoric acid is given below

- As we can see in the structure of ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ a single P−P bond is present. Also it contains two P=O bonds and four P−OH bonds .In the structure of ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ the P−O bonds have a bond length of 151 pm and P−P bond has a bond length of 219 pm.
- ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ is a mineral acid and in the solid state it is present as dihydrate which has the molecular formula${{H}_{4}}{{P}_{2}}{{O}_{6}}.2{{H}_{2}}O$. It contains oxonium ions and is formulated as${{\left[ {{H}_{3}}{{O}^{+}} \right]}_{_{2}}}{{\left[ {{H}_{2}}{{P}_{2}}{{O}_{6}} \right]}^{2-}}$.
From the above discussions it’s clear that the compound hypophosphoric acid ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ has a P−P bond in it.
Therefore, the answer is option (B). ${{H}_{4}}{{P}_{2}}{{O}_{6}}$.
Additional information:
The hypophosphoric acid is generally used as reducing agents, wetting agents, as a bleaching agent and as a stimulant in pharmaceutical agents.
Note: Keep in mind that the hypophosphoric acid is a triprotic acid. Also do not confuse Hypophosphorous acid with Hypophosphoric acid. Hypophosphorous acid or phosphinic acid has the molecular formula ${{H}_{3}}P{{O}_{2}}$ whereas Hypophosphoric acid has the molecular formula ${{H}_{4}}{{P}_{2}}{{O}_{6}}$. In addition to this, there are also other oxyacids of phosphorus such as Phosphorus acid, Diphosphoric acid (Pyrophosphoric acid), Peroxophosphoric acid etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




