
Which of the following compounds are antiaromatic?
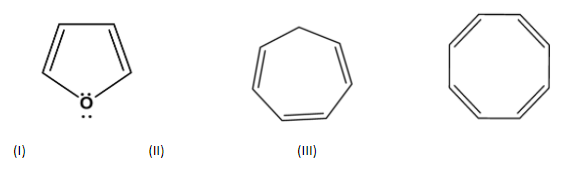
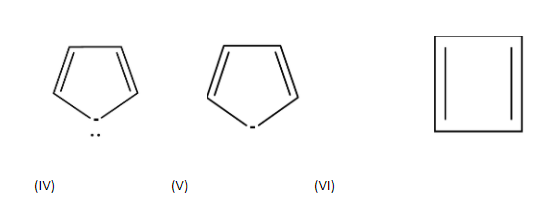


Answer
582.6k+ views
Hint: A compound is said to the anti-aromatic, if it is cyclic, planar, have the hybridization as $s{{p}^{2}}$and obeys the$(4n)\pi e{{s}^{-}}$rule in which n can be any integer such as 1,2,3,---and so on. From the above compounds, which satisfy this condition ,is called the anti -aromatic compound. now identify it.
Complete step by step answer:
First of all, we should know what aromatic compounds are. A compound is said to be aromatic if it satisfies the following conditions:
1. there should be $(4n+2)\pi e{{s}^{-}}$ and here, n can be any integer 1,2,3,3-----and so on.
2. Compounds should be cyclic( i.e. in ring) and must contain unhybridized p-orbitals and the ring should be planar .
3. All the carbon atoms should be $s{{p}^{2}}$ hybridized.
4. Cyclic systems should have conjugation ( i.e. there should be the delocalization of pi-electrons).
On the other hand, anti-aromatic compounds also satisfy all the conditions except the one, that there are $(4n)\pi e{{s}^{-}}$ instead of $(4n+2)\pi e{{s}^{-}}$. And the pi electrons are in the multiple of 4 like
4,8,12,16---and so on.
Note: in this, formula $(4n)\pi e{{s}^{-}}$, the value of cannot be negative and it always has a positive value and the compounds which doesn’t above any conditions or any one condition of the aromaticity, those compounds are said to be non-aromatic compounds.
Complete step by step answer:
First of all, we should know what aromatic compounds are. A compound is said to be aromatic if it satisfies the following conditions:
1. there should be $(4n+2)\pi e{{s}^{-}}$ and here, n can be any integer 1,2,3,3-----and so on.
2. Compounds should be cyclic( i.e. in ring) and must contain unhybridized p-orbitals and the ring should be planar .
3. All the carbon atoms should be $s{{p}^{2}}$ hybridized.
4. Cyclic systems should have conjugation ( i.e. there should be the delocalization of pi-electrons).
On the other hand, anti-aromatic compounds also satisfy all the conditions except the one, that there are $(4n)\pi e{{s}^{-}}$ instead of $(4n+2)\pi e{{s}^{-}}$. And the pi electrons are in the multiple of 4 like
4,8,12,16---and so on.
| S.no | Compound | Cyclic | Planar | $s{{p}^{2}}$hybridization | $(4n)\pi e{{s}^{-}}$ | Anti -aromatic |
| 1. | 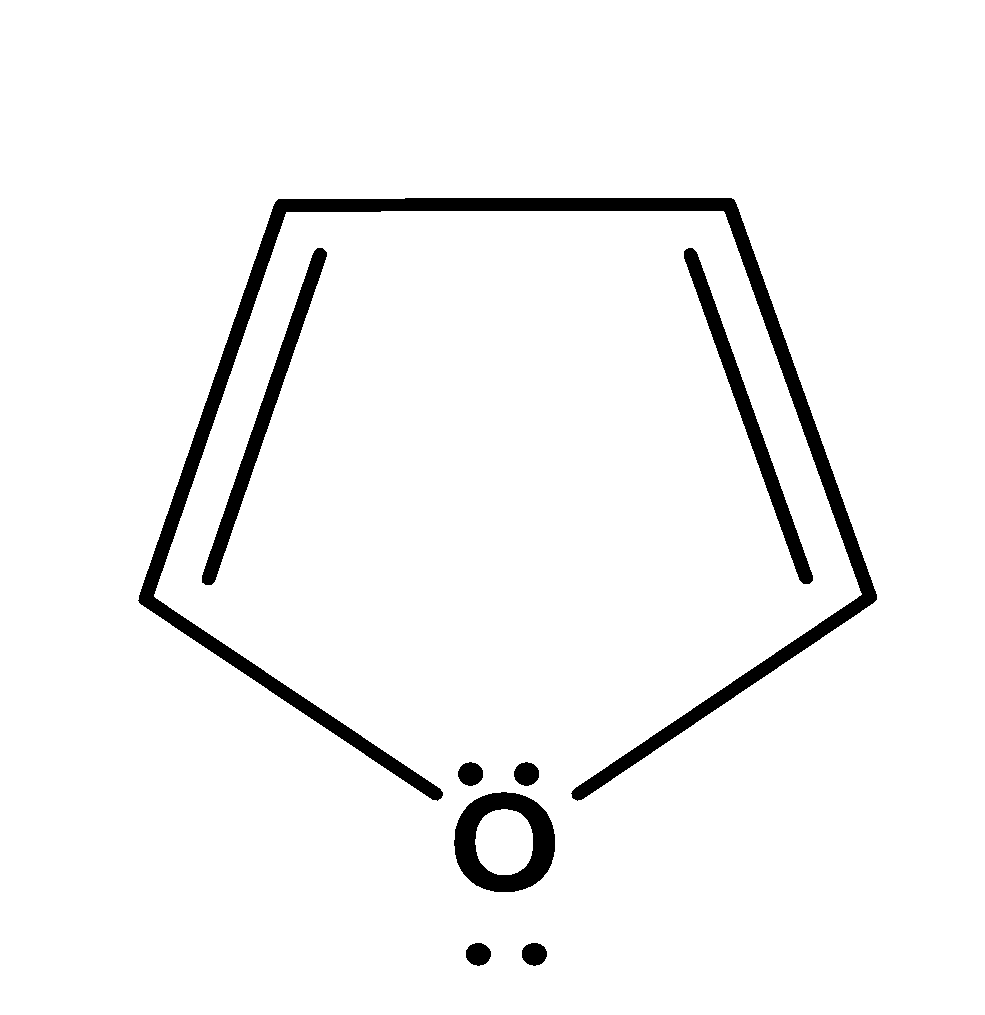
| cyclic | planar | $s{{p}^{2}}$ | Has 2 pi bonds i.e. 4 electrons (n=1) and 2 electrons of the lone pair which is inside the plane i.e. there are a total no of 6 electrons . so, it doesn’t obey this rule. | no |
| 2. | 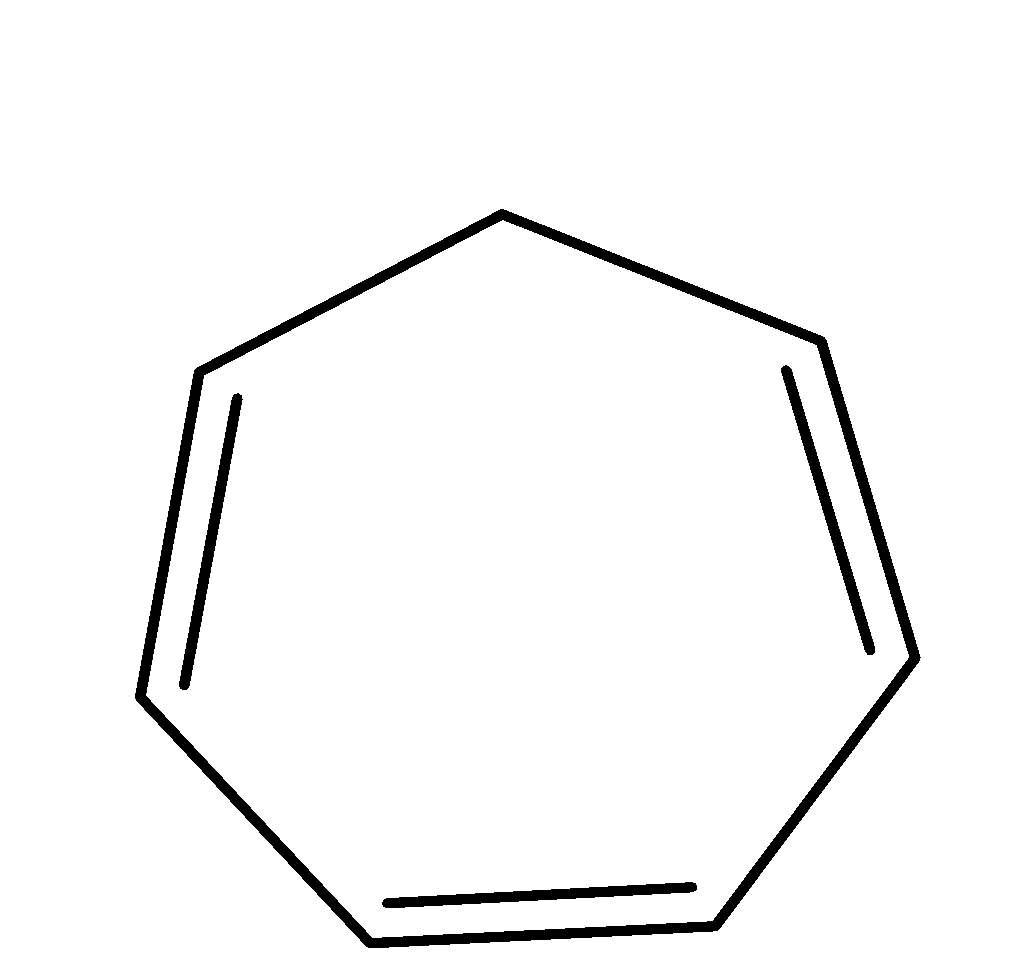
| cyclic | planar | One carbon is $s{{p}^{3}}$hybridized | Has 3 pi bonds i.e. 6 electrons=1 and it is not a multiple of 4 and doesn’t obey this rule. | no |
| 3. | 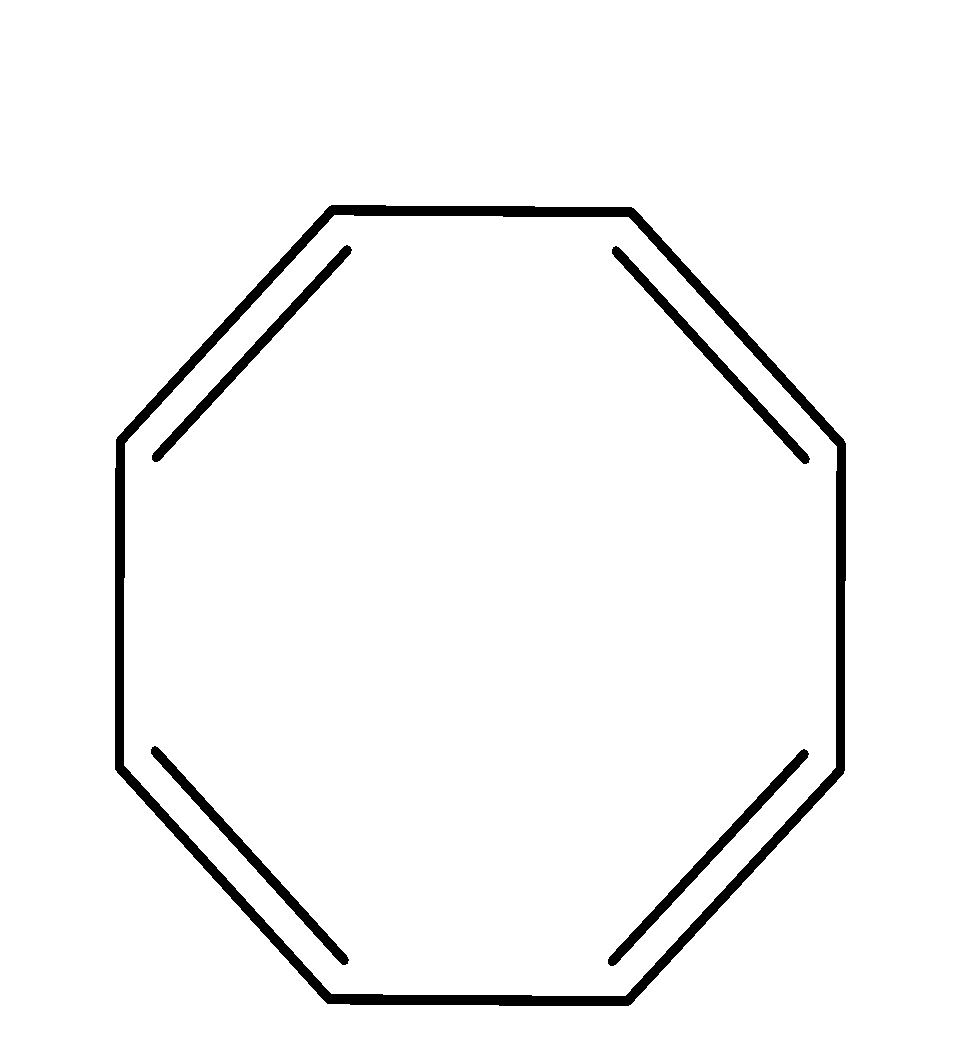
| cyclic | planar | $s{{p}^{2}}$ | Has 4 pi bonds i.e.8 electrons(n=2) and is multiple of 4. So, it obeys this rule. | yes |
| 4. | 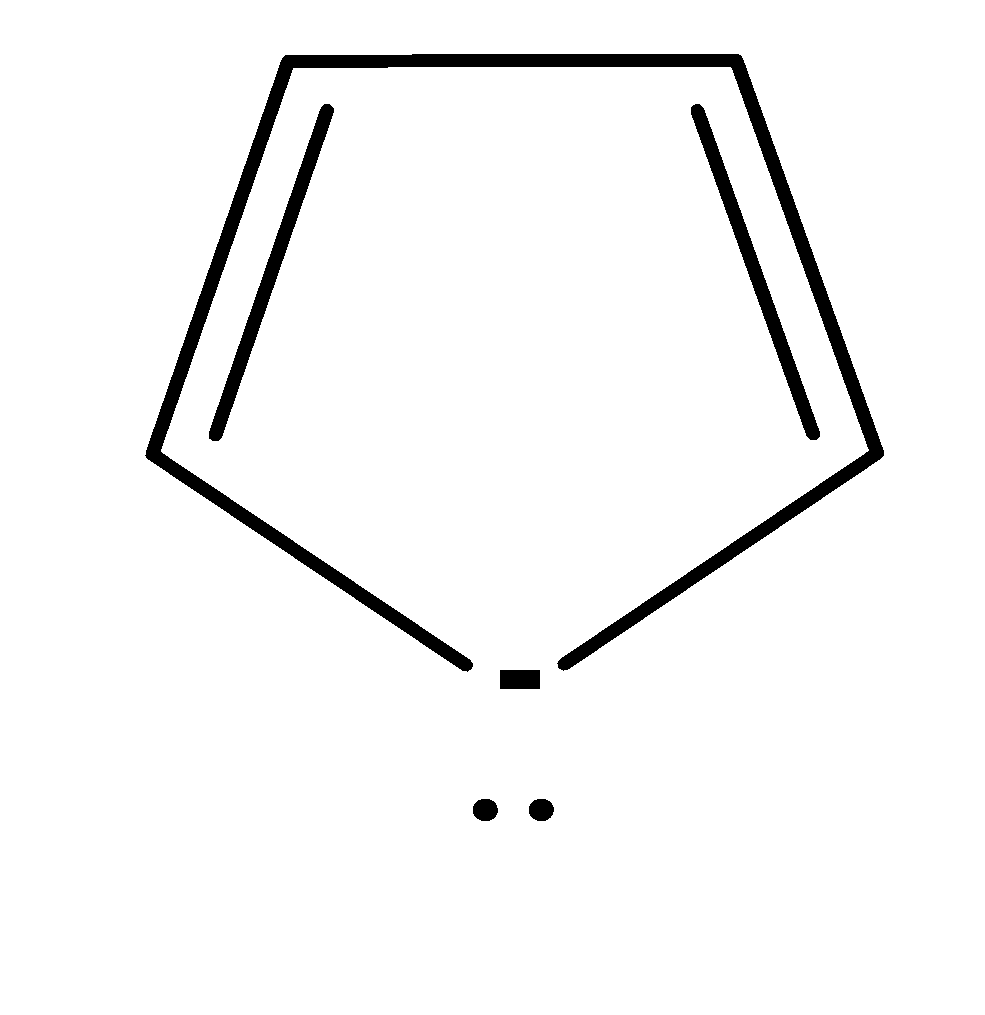
| Cyclic | planar | $s{{p}^{2}}$ | Has 2 pi bonds i.e. 4 electrons (n=1) and 1 electron of the negative charge and electrons of lone pair would not be considered because they are outside the plane i.e. there are a total no of 3 electrons . so, it doesn’t obey this rule. | no |
| 5. | 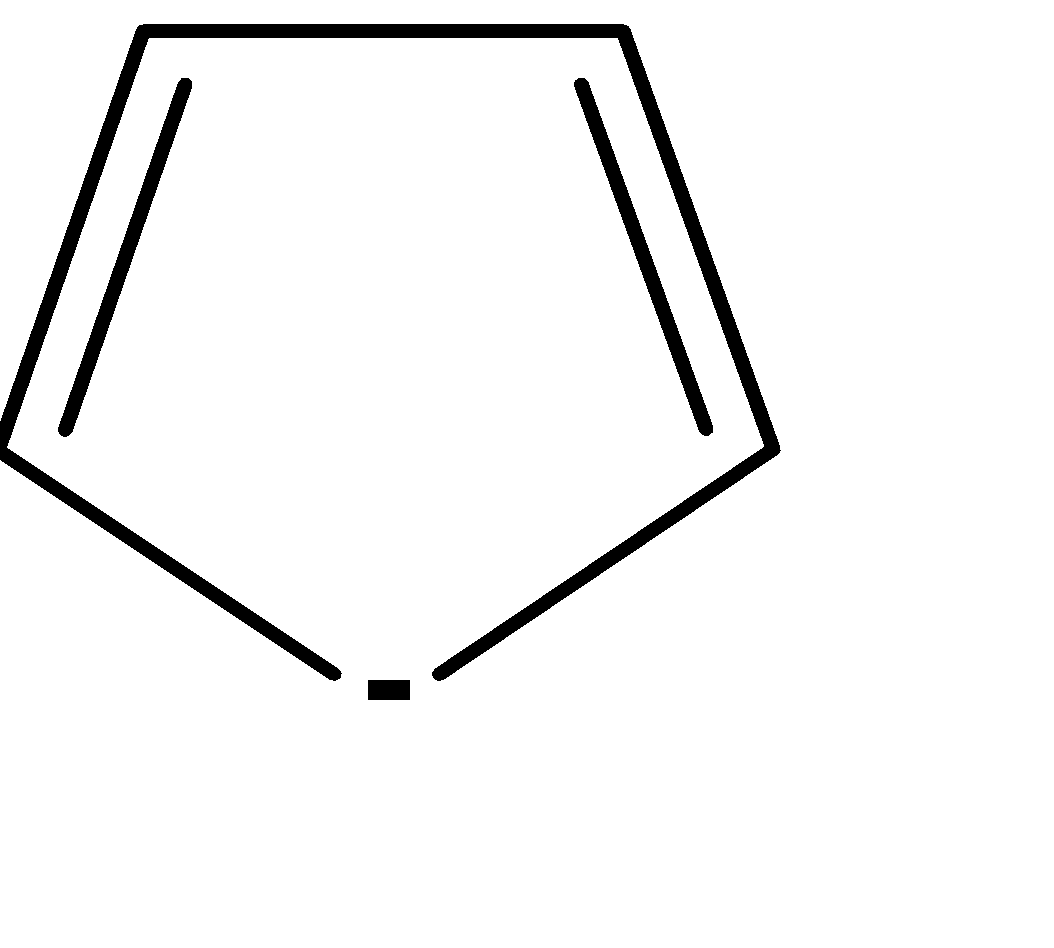
| Cyclic | planar | $s{{p}^{2}}$ | Has 2 pi bonds i.e. 4 electrons (n=1) and 2 electrons of the lone pair i.e. there are a total no of 6 electrons . so, it doesn’t obey this rule. | yes |
| 6. | 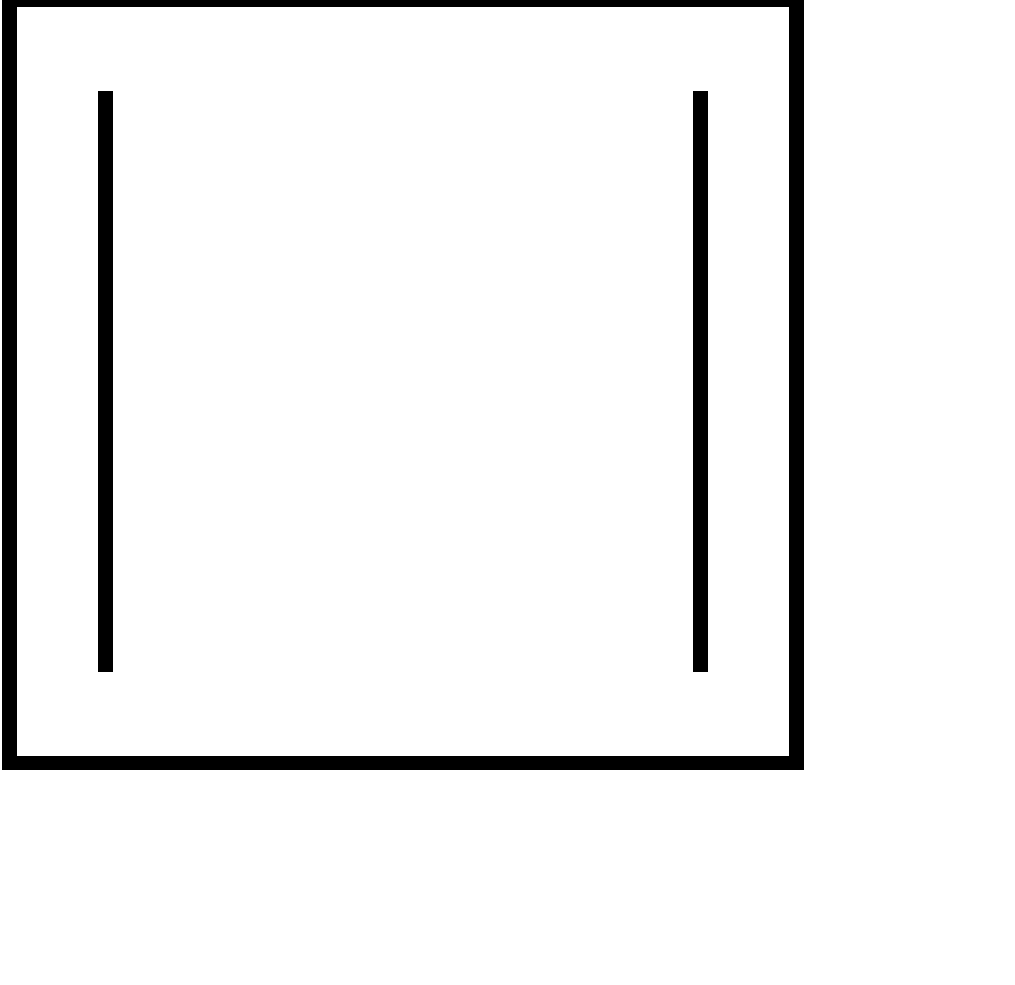
| cyclic | planar | $s{{p}^{2}}$ | Has 2 pi bonds i.e. 4 electrons (n=1) and is multiple of 4. So, obey this rule. | yes |
Note: in this, formula $(4n)\pi e{{s}^{-}}$, the value of cannot be negative and it always has a positive value and the compounds which doesn’t above any conditions or any one condition of the aromaticity, those compounds are said to be non-aromatic compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




