
Which of the following compounds are aromatic.
A.
B.
C.
D.




Answer
576.3k+ views
Hint:
Aromatic compounds are defined as characterized by the presence of one or more ring which are uniquely stable structures which means the $\pi $(pi) electrons of molecules have strong bonding arrangements. The compound must follow these four conditions which are
-The aromatic compound must be a cyclic compound.
-The aromatic compound has planarity i.e. $s{p^2}$ hybridization.
-Delocalization of $\pi $ electrons are also present in aromatic compounds.
-The aromatic compound followed the Huckel’s rule of aromaticity i.e. $\left( {4n + 2} \right)\pi $ electrons.
Complete answer:
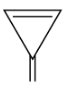
So, here in option (A), It is a cyclic compound and also has $s{p^2}$ hybridization in which planarity is present. There is $5\pi $ bond that is present in which $5 \times 2\pi $electrons are involved in the ring.
According to Huckel’s rule of aromaticity i.e. $\left( {4\pi + 2} \right)$$\pi $ electron …… (i)
Put the value of n in the equation (i)
In which, $n = 0 \to \left[ {4\left( 0 \right) + 2} \right] = 2\pi $electron pair
$n = 1 \to \left[ {4\left( 1 \right) + 2} \right] = 6\pi $ electron pair
$n = 2 \to \left[ {4\left( 2 \right) + 2} \right] = 10\pi $ electron pair
Here $10\pi $ electrons are matched to the value of $\pi $ electron of this compound. So, this compound is an aromatic compound.
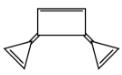
In the option (B), the given compound is cyclic compound and have $s{p^2}$ hybridization, the delocalization of $\pi $ electrons are also present and $4\pi $ bond occur which means $4 \times 2\pi $electron $ = 8\pi $ electrons. According to Huckel’s rule of aromaticity i.e. \[\left( {4n + 2} \right)\pi \]electrons are not applied in this compound due to the value of Huckel’s rule are not equal or match with the \[8\pi \] electron. So, this is not an aromatic compound.
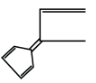
In the option (C), these are not cyclic compound and $s{p^2}$ hybridization is absent, delocalization of $\pi $ electron may occur in which $2\pi $ bond and these have $2 \times 2\pi $ electron which means $4\pi $ electron and their value is not equal to the Huckel’s rule of aromaticity. So, this compound does not follow the conditions of aromaticity.
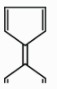
In the option (D), these are cyclic compounds and satisfy all conditions of aromaticity except one of them i.e. these do not follow the Huckel’s rule of aromaticity. The second five membered cyclic ring has an anion charge not cation. So, it loses aromaticity due to \[8\pi \] electrons, it is not an aromatic ring.
Therefore, (A) is the correct option because this compound is an aromatic compound which follows all the properties of aromaticity.
Note:
Huckel’s rule of aromaticity is the rule which determines that the ring is planar which has aromatic properties in the molecule. The cyclic molecules are considered aromatic if it has \[4n + 2\pi \] electrons.
Aromatic compounds are defined as characterized by the presence of one or more ring which are uniquely stable structures which means the $\pi $(pi) electrons of molecules have strong bonding arrangements. The compound must follow these four conditions which are
-The aromatic compound must be a cyclic compound.
-The aromatic compound has planarity i.e. $s{p^2}$ hybridization.
-Delocalization of $\pi $ electrons are also present in aromatic compounds.
-The aromatic compound followed the Huckel’s rule of aromaticity i.e. $\left( {4n + 2} \right)\pi $ electrons.
Complete answer:
So, here in option (A), It is a cyclic compound and also has $s{p^2}$ hybridization in which planarity is present. There is $5\pi $ bond that is present in which $5 \times 2\pi $electrons are involved in the ring.
According to Huckel’s rule of aromaticity i.e. $\left( {4\pi + 2} \right)$$\pi $ electron …… (i)
Put the value of n in the equation (i)
In which, $n = 0 \to \left[ {4\left( 0 \right) + 2} \right] = 2\pi $electron pair
$n = 1 \to \left[ {4\left( 1 \right) + 2} \right] = 6\pi $ electron pair
$n = 2 \to \left[ {4\left( 2 \right) + 2} \right] = 10\pi $ electron pair
Here $10\pi $ electrons are matched to the value of $\pi $ electron of this compound. So, this compound is an aromatic compound.
In the option (B), the given compound is cyclic compound and have $s{p^2}$ hybridization, the delocalization of $\pi $ electrons are also present and $4\pi $ bond occur which means $4 \times 2\pi $electron $ = 8\pi $ electrons. According to Huckel’s rule of aromaticity i.e. \[\left( {4n + 2} \right)\pi \]electrons are not applied in this compound due to the value of Huckel’s rule are not equal or match with the \[8\pi \] electron. So, this is not an aromatic compound.
In the option (C), these are not cyclic compound and $s{p^2}$ hybridization is absent, delocalization of $\pi $ electron may occur in which $2\pi $ bond and these have $2 \times 2\pi $ electron which means $4\pi $ electron and their value is not equal to the Huckel’s rule of aromaticity. So, this compound does not follow the conditions of aromaticity.
In the option (D), these are cyclic compounds and satisfy all conditions of aromaticity except one of them i.e. these do not follow the Huckel’s rule of aromaticity. The second five membered cyclic ring has an anion charge not cation. So, it loses aromaticity due to \[8\pi \] electrons, it is not an aromatic ring.
Therefore, (A) is the correct option because this compound is an aromatic compound which follows all the properties of aromaticity.
Note:
Huckel’s rule of aromaticity is the rule which determines that the ring is planar which has aromatic properties in the molecule. The cyclic molecules are considered aromatic if it has \[4n + 2\pi \] electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




