
Which of the following compound has DBE equals to benzene
A.
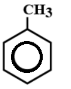
B.
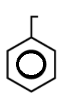
C.
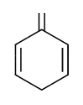
D. All the above



Answer
533.4k+ views
Hint: To solve this question we should first know about the Double bond equivalent of the Benzene. The Double bond equivalent of benzene is 4. Now, we have to find the DBE of all the structures and see which structure has the same DBE as that of the benzene.
Complete step by step answer: An unsaturated molecule is a molecule that forms one double or triple bond between carbon atoms. Some of them are alkenes, alkynes, or aromatic hydrocarbons.
A saturated molecule is a molecule that has no rings and no double bonds between carbon atoms. In saturated compounds, each carbon atom forms a bond with hydrogen.
Double bond equivalent (DBE) is the calculation of the number of unsaturated bonds which is there in an organic compound. A molecule having a double bond or a ring is considered to be an Unsaturated molecule.
$Degree\,of\,Unsaturation\,=\,C+1\,-\,\dfrac{H}{2}\,\,-\,\dfrac{X}{2}\,+\,\dfrac{N}{2}$
C= Number of Carbon atoms present
H= Number of hydrogen atoms present
X= Number of halogen atoms present
N= Number of Nitrogen atom present
BENZENE: A ring that has six carbon atoms that are bonded by single and double bonds arranged alternatively. The molecular formula of it is ${{C}_{6}}{{H}_{6}}$.
The molecular formula for benzene is C6H6. Thus,
In Benzene,
Carbon atoms = 6
N= 0
X= 0
H= 6
Therefore,
Degree of Unsaturation of benzene = 4
$Double\,Bond\,Equivalent\,=\,Number\,of\,double\,bonds\,+\,Number\,of\,rings$
In Benzene, There are 3 double bonds and 1 Ring DBE
So, DBE of Benzene = 3 + 1
= 4
In this, There are 3 double bonds and 1 Ring DBE
DBE = 3+1
= 4
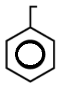
In this, There are 3 double bonds and 1 Ring DBE
DBE = 3+1
= 4
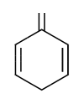
The DBE of all the structures are 4 which is equivalent to the benzene.
So Option (D) All the above is correct.
Note: The presence of oxygen and sulphur does not affect the degree of unsaturation or calculation of double bond equivalent in any compound. DBE helps in the calculation of the number of $\pi $bonds and the number of cyclic rings.
Complete step by step answer: An unsaturated molecule is a molecule that forms one double or triple bond between carbon atoms. Some of them are alkenes, alkynes, or aromatic hydrocarbons.
A saturated molecule is a molecule that has no rings and no double bonds between carbon atoms. In saturated compounds, each carbon atom forms a bond with hydrogen.
Double bond equivalent (DBE) is the calculation of the number of unsaturated bonds which is there in an organic compound. A molecule having a double bond or a ring is considered to be an Unsaturated molecule.
$Degree\,of\,Unsaturation\,=\,C+1\,-\,\dfrac{H}{2}\,\,-\,\dfrac{X}{2}\,+\,\dfrac{N}{2}$
C= Number of Carbon atoms present
H= Number of hydrogen atoms present
X= Number of halogen atoms present
N= Number of Nitrogen atom present
BENZENE: A ring that has six carbon atoms that are bonded by single and double bonds arranged alternatively. The molecular formula of it is ${{C}_{6}}{{H}_{6}}$.
The molecular formula for benzene is C6H6. Thus,
In Benzene,
Carbon atoms = 6
N= 0
X= 0
H= 6
Therefore,
Degree of Unsaturation of benzene = 4
$Double\,Bond\,Equivalent\,=\,Number\,of\,double\,bonds\,+\,Number\,of\,rings$
In Benzene, There are 3 double bonds and 1 Ring DBE
So, DBE of Benzene = 3 + 1
= 4

In this, There are 3 double bonds and 1 Ring DBE
DBE = 3+1
= 4

In this, There are 3 double bonds and 1 Ring DBE
DBE = 3+1
= 4

The DBE of all the structures are 4 which is equivalent to the benzene.
So Option (D) All the above is correct.
Note: The presence of oxygen and sulphur does not affect the degree of unsaturation or calculation of double bond equivalent in any compound. DBE helps in the calculation of the number of $\pi $bonds and the number of cyclic rings.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




