
Which of the following combinations of bond pair (B.P) and lone pair (L.P) give the same shape?
(i) 3 B.P + 1 L.P
(ii) 2 B.P + 2 L.P
(iii) 2 B.P + 1 L.P
(iv) 2 B.P + 0 L.P
A) ii) and iii)
B) i) and iv)
C) iv) and ii)
D) iii) and iv)
Answer
478.5k+ views
Hint: To solve this question we need to find the hybridisation with the help of the no. of bond pairs and lone pairs. Lone pairs occupy one side of the geometry of the molecule. The Lone Pairs repulsion causes the bond angle to change. Hence the shape of the molecule will be different from the geometry.
Complete answer:
To find the hybridization we need to add up the no. of bond pairs and no. of lone pairs. If the answer obtained is:
Let us note the shape of the molecules one by one:
i) 3 B.P + 1 L.P: The sum is $ 3 + 1 = 4 \to s{p^3} $ . The geometry of the molecule is Tetrahedral. It has 3 Bond Pairs and 1 lone pair. One of the bonds of tetrahedral is occupied with Lone Pair. The shape can be given as:

Lone Pair occupies one bond of the tetrahedron. The shape of the molecule thus becomes trigonal pyramidal.
ii) 2 B.P + 2 L.P : The sum is $ 2 + 2 = 4 \to s{p^3} $ . The geometry of the molecule is Tetrahedral. It has 2 Bond Pairs and 2 lone pairs. Two of the bonds of tetrahedral are occupied with Lone Pairs. The shape can be given as:
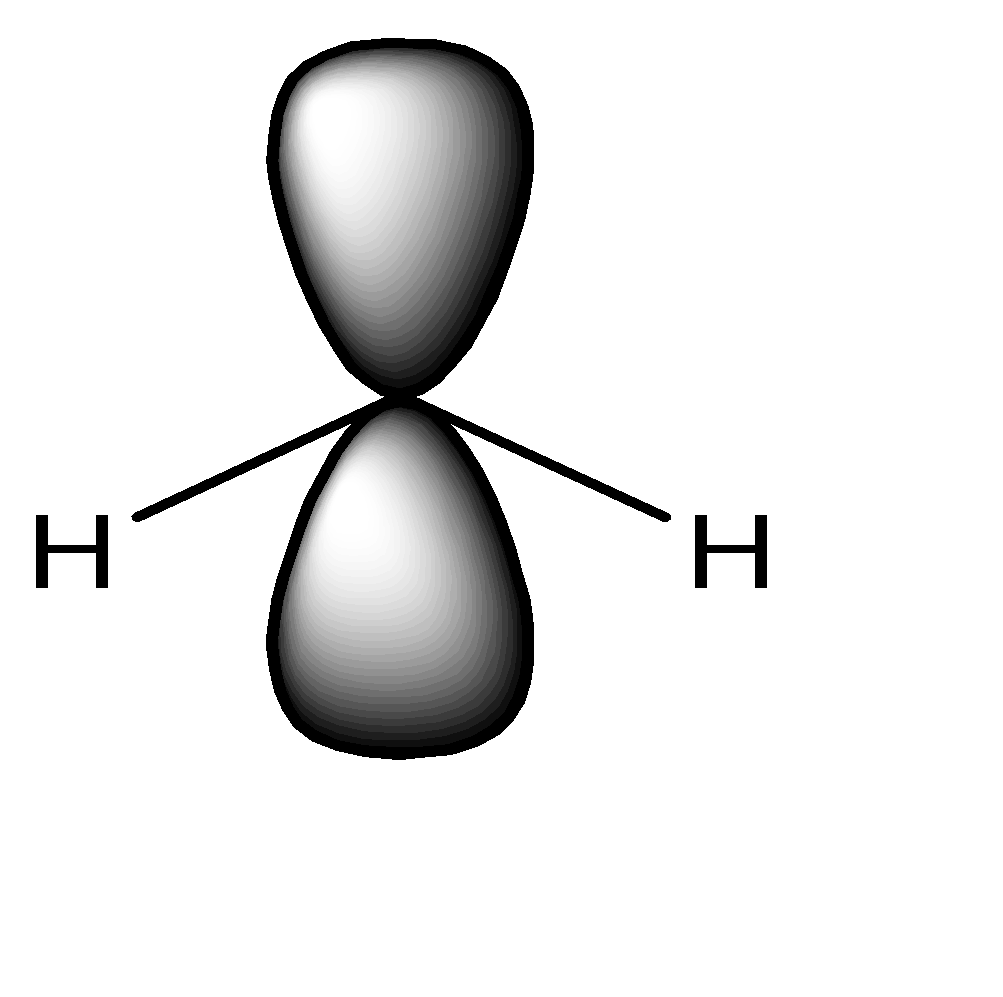
Lone Pair occupies two bonds of the tetrahedron. The shape of the molecule thus becomes angular.
iii) 2 B.P + 1 L.P: The sum is $ 2 + 1 = 3 \to s{p^2} $ . The geometry of the molecule is Trigonal Planar. It has 2 Bond Pairs and 1 lone pair. Two of the bonds of Trigonal Planar are occupied with Lone Pair. The shape can be given as:

Lone Pair occupies two bonds of the trigonal planar. The shape of the molecule thus becomes angular.
iv) 2 B.P + 0 L.P: The sum is $ 2 + 0 = 2 \to sp $ . The geometry of the molecule is Linear. It has 2 Bond Pairs and 0 lone pairs. No bonds of Linear geometry are occupied with Lone Pair. The shape can be given as:
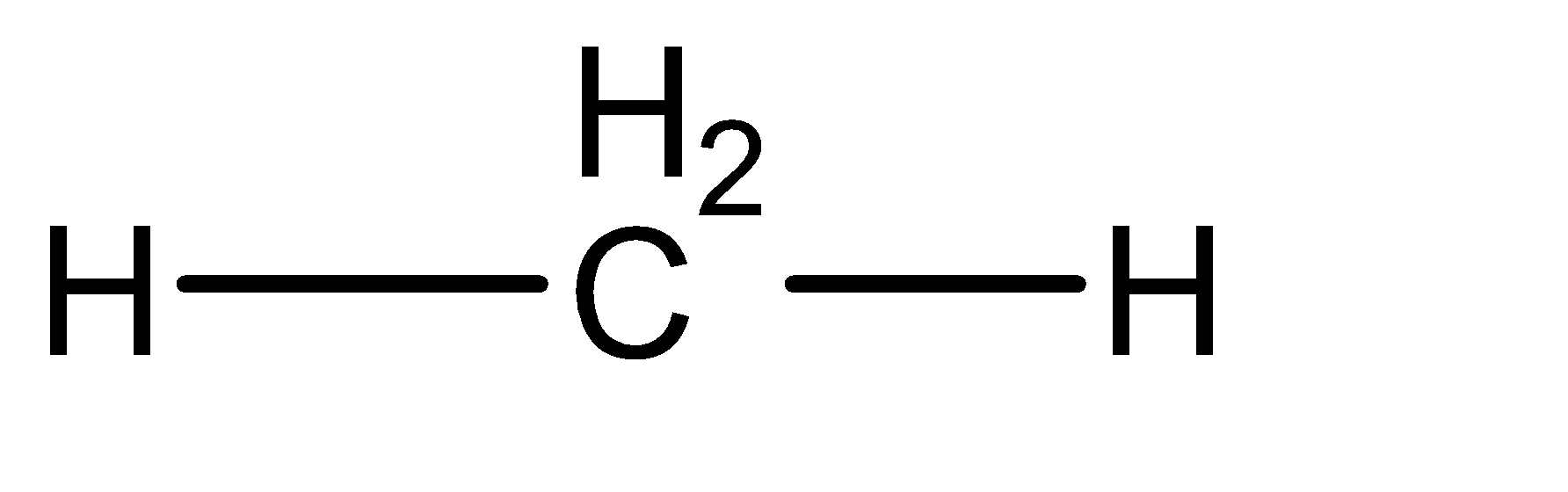
As there are no lone pairs the shape of the molecule will also be linear.
ii) and iii) have the same shape.
Hence the correct answer is option A).
Note:
If the compounds contain no Lone Pairs, the geometry and shape of the molecules are the same. If the geometry is sp, it will not contain any lone pairs. The hybridisation of polyatomic compounds can be given by the formula:
$ Hybridisation = \dfrac{{no.of{\text{ }}valence{\text{ }}electron\operatorname{s} {\text{ }}in{\text{ }}the{\text{ }}central{\text{ }}atom + no.ofHydrogen{\text{ }}Atoms + no.of{\text{ }}Halide{\text{ }}Atoms \pm Formal{\text{ }}Ch\arg e}}{2} $ .
Complete answer:
To find the hybridization we need to add up the no. of bond pairs and no. of lone pairs. If the answer obtained is:
| Answer Obtained/Sum of BP+ LP | Hybridisation | Geometry |
| 2 | sp | Linear |
| 3 | $ s{p^2} $ | Trigonal Planar |
| 4 | $ s{p^3} $ | Tetrahedral |
| 5 | $ s{p^3}d $ | Trigonal Bipyramidal |
| 6 | $ s{p^3}{d^2} $ | Octahedral |
Let us note the shape of the molecules one by one:
i) 3 B.P + 1 L.P: The sum is $ 3 + 1 = 4 \to s{p^3} $ . The geometry of the molecule is Tetrahedral. It has 3 Bond Pairs and 1 lone pair. One of the bonds of tetrahedral is occupied with Lone Pair. The shape can be given as:

Lone Pair occupies one bond of the tetrahedron. The shape of the molecule thus becomes trigonal pyramidal.
ii) 2 B.P + 2 L.P : The sum is $ 2 + 2 = 4 \to s{p^3} $ . The geometry of the molecule is Tetrahedral. It has 2 Bond Pairs and 2 lone pairs. Two of the bonds of tetrahedral are occupied with Lone Pairs. The shape can be given as:

Lone Pair occupies two bonds of the tetrahedron. The shape of the molecule thus becomes angular.
iii) 2 B.P + 1 L.P: The sum is $ 2 + 1 = 3 \to s{p^2} $ . The geometry of the molecule is Trigonal Planar. It has 2 Bond Pairs and 1 lone pair. Two of the bonds of Trigonal Planar are occupied with Lone Pair. The shape can be given as:

Lone Pair occupies two bonds of the trigonal planar. The shape of the molecule thus becomes angular.
iv) 2 B.P + 0 L.P: The sum is $ 2 + 0 = 2 \to sp $ . The geometry of the molecule is Linear. It has 2 Bond Pairs and 0 lone pairs. No bonds of Linear geometry are occupied with Lone Pair. The shape can be given as:
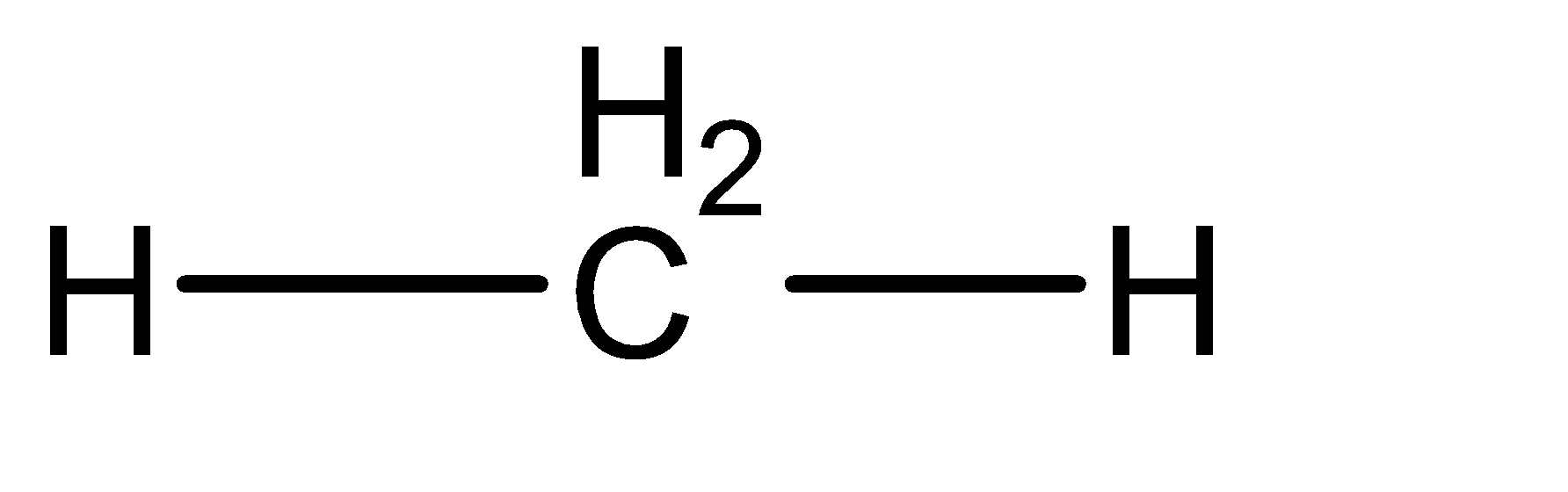
As there are no lone pairs the shape of the molecule will also be linear.
ii) and iii) have the same shape.
Hence the correct answer is option A).
Note:
If the compounds contain no Lone Pairs, the geometry and shape of the molecules are the same. If the geometry is sp, it will not contain any lone pairs. The hybridisation of polyatomic compounds can be given by the formula:
$ Hybridisation = \dfrac{{no.of{\text{ }}valence{\text{ }}electron\operatorname{s} {\text{ }}in{\text{ }}the{\text{ }}central{\text{ }}atom + no.ofHydrogen{\text{ }}Atoms + no.of{\text{ }}Halide{\text{ }}Atoms \pm Formal{\text{ }}Ch\arg e}}{2} $ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




