
Which of the following carbonyl compound(s) form (s) readily isolable hydrate?
A.

B.
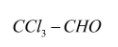
C.

D.


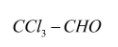


Answer
570k+ views
Hint: As it is already known that the carbonyl compounds involving aldehydes and ketones react readily with water to give gem-diols or alcoholic compounds which are more stable than the parent compound.
Complete step by step solution: As we know that when a carbonyl compound and water are present together, an equilibrium is set between the carbonyl compound and the corresponding gem-diol. The compound will be resonance stable. So the compounds having ketone and aldehyde will form isolable hydrates. The addition of a strong electron withdrawing group destabilizes the carbonyl and tends to form stable gem-diols like in our first $1,2,3 - Indantrione$ and second the chloral compound. The hydroxide ion acts as a nucleophile and attacks the carbonyl carbons which shift the double bond between carbon and oxygen and make the oxygen partially negative, now this oxygen can proceed to protonation of the so formed alkoxide and results in formation of diols.

Similarly the condition can be seen in chloral as:

In the third compound also there is diol formation:

Poly-vicinal carbonyl compounds generally exist as hydrates in the presence of water and we can easily see that the $C = O$ bond in such compounds is highly polarized with the terminal carbons being the positive ends. But the equilibrium in these compounds like the acetone and water may be shifted towards either of the compounds, thus it does not form readily isolable hydrate.
Hence, from the above explanation we can that the correct answers are (A), (B) and (C).
Note: Chloral hydrate has a characteristic property of being a sedative and therefore it can be added to alcoholic beverages to generate a knockout drink and Ninhydrin is generally used as a spray in forensics to identify the finger prints. Formaldehyde is also an exception of isolable hydrates as the weak pi-bonds of carbonyl compound and size of hydrogen substituents favours addition.
Complete step by step solution: As we know that when a carbonyl compound and water are present together, an equilibrium is set between the carbonyl compound and the corresponding gem-diol. The compound will be resonance stable. So the compounds having ketone and aldehyde will form isolable hydrates. The addition of a strong electron withdrawing group destabilizes the carbonyl and tends to form stable gem-diols like in our first $1,2,3 - Indantrione$ and second the chloral compound. The hydroxide ion acts as a nucleophile and attacks the carbonyl carbons which shift the double bond between carbon and oxygen and make the oxygen partially negative, now this oxygen can proceed to protonation of the so formed alkoxide and results in formation of diols.

Similarly the condition can be seen in chloral as:

In the third compound also there is diol formation:

Poly-vicinal carbonyl compounds generally exist as hydrates in the presence of water and we can easily see that the $C = O$ bond in such compounds is highly polarized with the terminal carbons being the positive ends. But the equilibrium in these compounds like the acetone and water may be shifted towards either of the compounds, thus it does not form readily isolable hydrate.
Hence, from the above explanation we can that the correct answers are (A), (B) and (C).
Note: Chloral hydrate has a characteristic property of being a sedative and therefore it can be added to alcoholic beverages to generate a knockout drink and Ninhydrin is generally used as a spray in forensics to identify the finger prints. Formaldehyde is also an exception of isolable hydrates as the weak pi-bonds of carbonyl compound and size of hydrogen substituents favours addition.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




