
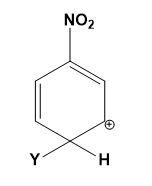
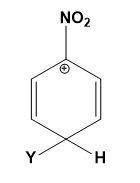
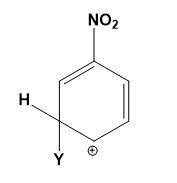
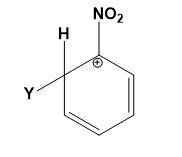
Which of the following carbocations is expected to be most stable?
(A)
(B)
(C)
(D)




Answer
577.8k+ views
Hint: The carbocation is the substance bearing a positive charge on it i.e. they are electron deficient species. They are primarily stabilised by electron donating groups like -O, alkyl, etc.
Complete step by step solution:
Let us study the $-N{{O}_{2}}$ group and the stability of carbocations;
-The $-N{{O}_{2}}$ group on the benzene deactivates the ring towards the electrophilic attacks. The presence of the $-N{{O}_{2}}$ group increases the positive charge densities on ortho and para positions of the benzene ring. Thus, it is a meta position directing group.
-All the four options are possible to be formed when an electrophile attacks the nitrobenzene depending on the position of attack. But the most stable compound is formed when the attack is on meta position as compared to ortho and para positions.
-The positive charge density is least at meta position thus, it makes the attack of electrophile easy as compared to ortho and para positions where the positive charge density is high.
-Therefore, the carbocations in which the meta position is occupied by an electrophile and ortho or para position occupied by positive charge is most stable. This can be explained as,
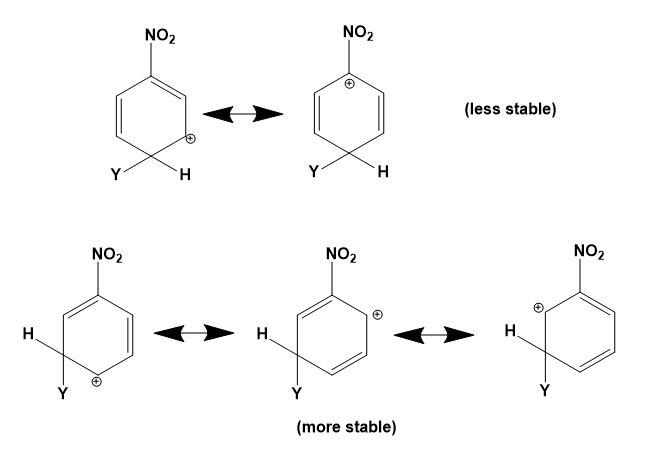
Therefore, option (C) is correct.
Note: Do note that option (A) and (B) can never be the answer, they are the most unstable carbocations. Instead of option (C) the other options which can be declared stable must consist of the other two resonating structures from the diagram given above.
Complete step by step solution:
Let us study the $-N{{O}_{2}}$ group and the stability of carbocations;
-The $-N{{O}_{2}}$ group on the benzene deactivates the ring towards the electrophilic attacks. The presence of the $-N{{O}_{2}}$ group increases the positive charge densities on ortho and para positions of the benzene ring. Thus, it is a meta position directing group.
-All the four options are possible to be formed when an electrophile attacks the nitrobenzene depending on the position of attack. But the most stable compound is formed when the attack is on meta position as compared to ortho and para positions.
-The positive charge density is least at meta position thus, it makes the attack of electrophile easy as compared to ortho and para positions where the positive charge density is high.
-Therefore, the carbocations in which the meta position is occupied by an electrophile and ortho or para position occupied by positive charge is most stable. This can be explained as,

Therefore, option (C) is correct.
Note: Do note that option (A) and (B) can never be the answer, they are the most unstable carbocations. Instead of option (C) the other options which can be declared stable must consist of the other two resonating structures from the diagram given above.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




