
Which of the following can’t be used in Friedel-Crafts reaction?
A) $ {\text{FeC}}{{\text{l}}_3} $
B) $ {\text{SnC}}{{\text{l}}_4} $
C) $ {\text{AlC}}{{\text{l}}_3} $
D) $ {\text{NaCl}} $
Answer
537.6k+ views
Hint :The Friedel Craft reaction takes place in presence of Lewis acid. Lewis acid is electron deficient in nature. $ {\text{FeC}}{{\text{l}}_3} $ , $ {\text{SnC}}{{\text{l}}_4} $ and $ {\text{AlC}}{{\text{l}}_3} $ act as Lewis acid in Friedel Craft reaction.
Complete Step By Step Answer:
Friedel craft reaction takes place in presence of Lewis acid. Lewis acid is an electron-pair acceptor which means it has to have a vacant orbital to accept those electrons.
$ {\text{FeC}}{{\text{l}}_3} $ , $ {\text{SnC}}{{\text{l}}_4} $ and $ {\text{AlC}}{{\text{l}}_3} $ act as Lewis acid as explained below-
The $ {\text{Cl}} $ atom due to high electronegativity reduces electron density from central atom
The central atom has a vacant orbital so it acts as an electrophile and attacks electron rich species.
This replaces the aromatic proton and the number of carbon atoms in the product formed is increased.
$ {\text{NaCl}} $ does not act as Lewis acid because it forms cation $ {\text{N}}{{\text{a}}^ + } $ which has very less tendency to accept electrons , as it has inert gas configuration.
Hence, the correct answer is ‘D’.
Additional Information:
Friedel craft reaction is of two types-
1. Friedel craft alkylation reaction- In this, aromatic ring reacts with alkyl halide in presence of Lewis acid to form alkyl aromatic compound. The reaction can be written as-
$ \Rightarrow $ $ {\text{Aromatic ring + Alkyl Halide}}\xrightarrow{{{\text{Lewis acid}}}}{\text{Alkyl aromatic compound}} $
Example-

Here the aromatic ring is benzene.
2. Friedel craft acylation reaction- In this reaction, addition of acyl group takes place in presence of Lewis acid. The reaction can be written as-
$ \Rightarrow $ $ {\text{Aromatic ring + RCOX}}\xrightarrow{{{\text{Lewis acid}}}}{\text{Acyl aromatic compound}} $
Example-
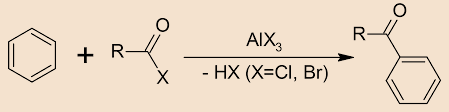
Here, the aromatic ring is benzene.
Note :
In Friedel craft reaction, the Lewis acid is used as it helps in the formation of carbocation which is an electrophile. This electrophile replaces the aromatic proton and increases the number of carbon atoms in the product during conversions.
Complete Step By Step Answer:
Friedel craft reaction takes place in presence of Lewis acid. Lewis acid is an electron-pair acceptor which means it has to have a vacant orbital to accept those electrons.
$ {\text{FeC}}{{\text{l}}_3} $ , $ {\text{SnC}}{{\text{l}}_4} $ and $ {\text{AlC}}{{\text{l}}_3} $ act as Lewis acid as explained below-
The $ {\text{Cl}} $ atom due to high electronegativity reduces electron density from central atom
The central atom has a vacant orbital so it acts as an electrophile and attacks electron rich species.
This replaces the aromatic proton and the number of carbon atoms in the product formed is increased.
$ {\text{NaCl}} $ does not act as Lewis acid because it forms cation $ {\text{N}}{{\text{a}}^ + } $ which has very less tendency to accept electrons , as it has inert gas configuration.
Hence, the correct answer is ‘D’.
Additional Information:
Friedel craft reaction is of two types-
1. Friedel craft alkylation reaction- In this, aromatic ring reacts with alkyl halide in presence of Lewis acid to form alkyl aromatic compound. The reaction can be written as-
$ \Rightarrow $ $ {\text{Aromatic ring + Alkyl Halide}}\xrightarrow{{{\text{Lewis acid}}}}{\text{Alkyl aromatic compound}} $
Example-

Here the aromatic ring is benzene.
2. Friedel craft acylation reaction- In this reaction, addition of acyl group takes place in presence of Lewis acid. The reaction can be written as-
$ \Rightarrow $ $ {\text{Aromatic ring + RCOX}}\xrightarrow{{{\text{Lewis acid}}}}{\text{Acyl aromatic compound}} $
Example-
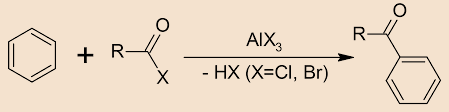
Here, the aromatic ring is benzene.
Note :
In Friedel craft reaction, the Lewis acid is used as it helps in the formation of carbocation which is an electrophile. This electrophile replaces the aromatic proton and increases the number of carbon atoms in the product during conversions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




