
Which of the following cannot be obtained as the only product in Wurtz’s coupling reaction of halides with sodium metal in ether solution?
A.
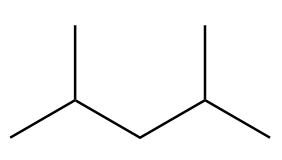
B.
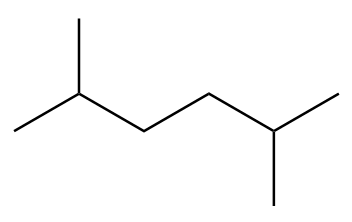
C.
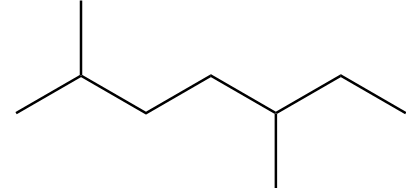
D. (A) and (C)



Answer
514.2k+ views
Hint : Wurtz reaction is a type of coupling reaction in which two moles of alkyl halide reacts with two moles of sodium metal in the presence of dry ether to yield higher alkanes of even number i.e., to form alkanes having even number of carbon atoms. The mechanism involves the formation of an alkyl radical followed by nucleophilic substitution reaction.
Complete Step By Step Answer:
When two same alkyl halides participate in the Wurtz reaction, then the alkane with even number of carbon atoms is formed and it is the only product of the reaction but if in case two different alkyl halides participate in the reaction, then there is possibility of formation of more than one product. Hence, in the Wurtz reaction an alkane with an odd number of carbon atoms will never be formed as the only product after the reaction. So, among given options the structures of alkane are given as follows:
Structure-A:
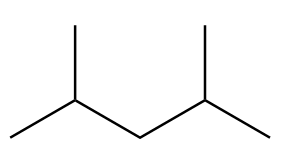
Number of carbon atoms in the chain $ = 5 $
As the alkane consists of an odd number of carbon atoms, it will not be the only product in Wurtz's reaction.
Structure-B:
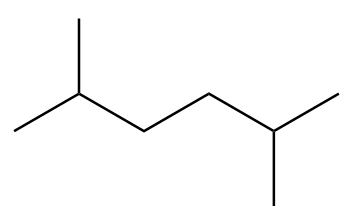
Number of carbon atoms in the chain $ = 6 $
As the alkane consists of an even number of carbon atoms, it will be the only product in the Wurtz’s reaction.
Structure-C:
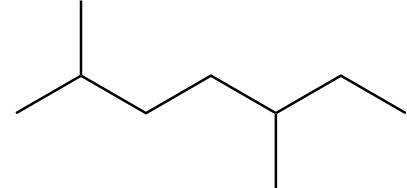
Number of carbon atoms in the chain $ = 7 $
As the alkane consists of an odd number of carbon atoms, it will not be the only product in Wurtz's reaction.
Hence, structures A and C will not be formed as the only product in Wurtz’s reaction.
So, option (D) is the correct answer.
Note :
It is important to note that other than sodium, other metals like silver, zinc, iron and copper can also be used for this reaction. If aryl halides are considered as reactants instead of alkyl halides, then the reaction is named as Wurtz-Fittig reaction.
Complete Step By Step Answer:
When two same alkyl halides participate in the Wurtz reaction, then the alkane with even number of carbon atoms is formed and it is the only product of the reaction but if in case two different alkyl halides participate in the reaction, then there is possibility of formation of more than one product. Hence, in the Wurtz reaction an alkane with an odd number of carbon atoms will never be formed as the only product after the reaction. So, among given options the structures of alkane are given as follows:
Structure-A:

Number of carbon atoms in the chain $ = 5 $
As the alkane consists of an odd number of carbon atoms, it will not be the only product in Wurtz's reaction.
Structure-B:

Number of carbon atoms in the chain $ = 6 $
As the alkane consists of an even number of carbon atoms, it will be the only product in the Wurtz’s reaction.
Structure-C:

Number of carbon atoms in the chain $ = 7 $
As the alkane consists of an odd number of carbon atoms, it will not be the only product in Wurtz's reaction.
Hence, structures A and C will not be formed as the only product in Wurtz’s reaction.
So, option (D) is the correct answer.
Note :
It is important to note that other than sodium, other metals like silver, zinc, iron and copper can also be used for this reaction. If aryl halides are considered as reactants instead of alkyl halides, then the reaction is named as Wurtz-Fittig reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




