
Which of the following can be used as dehydrating agents?
a.) Conc. $H_{ 2 }SO_{ 4 }$
b.) $POCl_{ 3 }$
c.) $P_{ 2 }O_{ 5 }$
d.) All of the above
Answer
548.7k+ views
Hint: To answer this question, you should know about dehydrating agents. Dehydrating agents are chemical compounds that can completely remove the water molecules from other substances. Now try to answer this accordingly.
Complete step by step answer:
Let’s discuss all the options one by one to reach the correct answer -
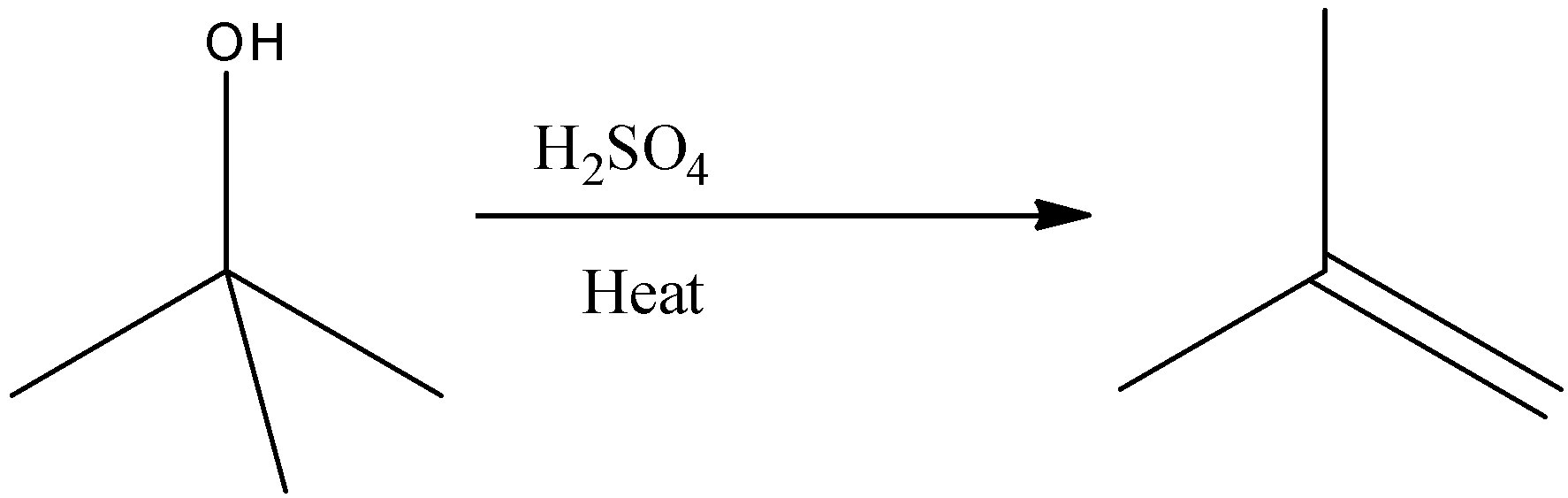
Option A, Conc. $H_{ 2 }SO_{ 4 }$ (Concentrated sulphuric acid) has a greater affinity to water. Hence it can remove water molecules from other substances. That’s why it is called a dehydrating agent.
Example - Dehydration of an alcohol
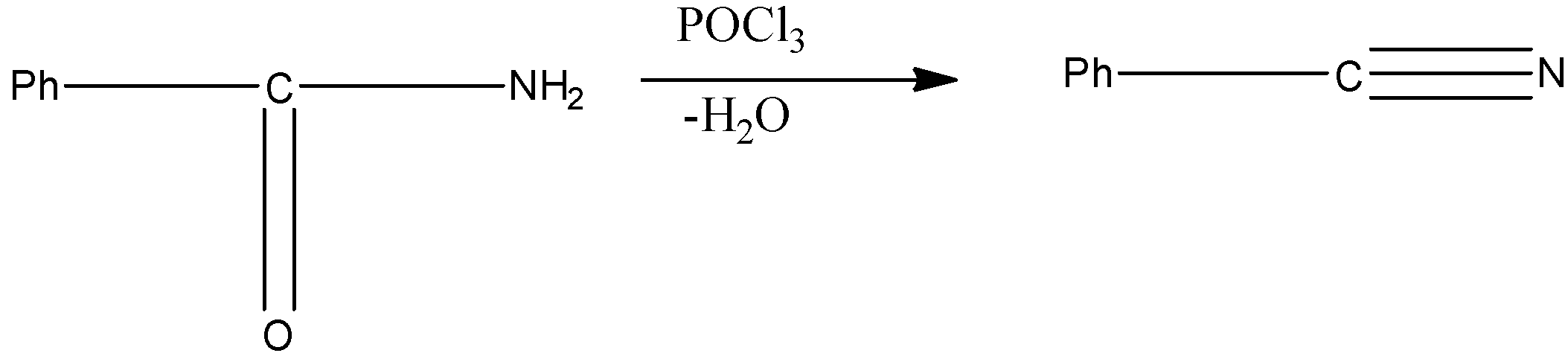
Option B, $POCl_{ 3 }$ is a commonly used dehydrating agent in chemical laboratories. It is used as a dehydrating agent in the preparation of nitriles from primary amides.
Example - Dehydration of Benzamide
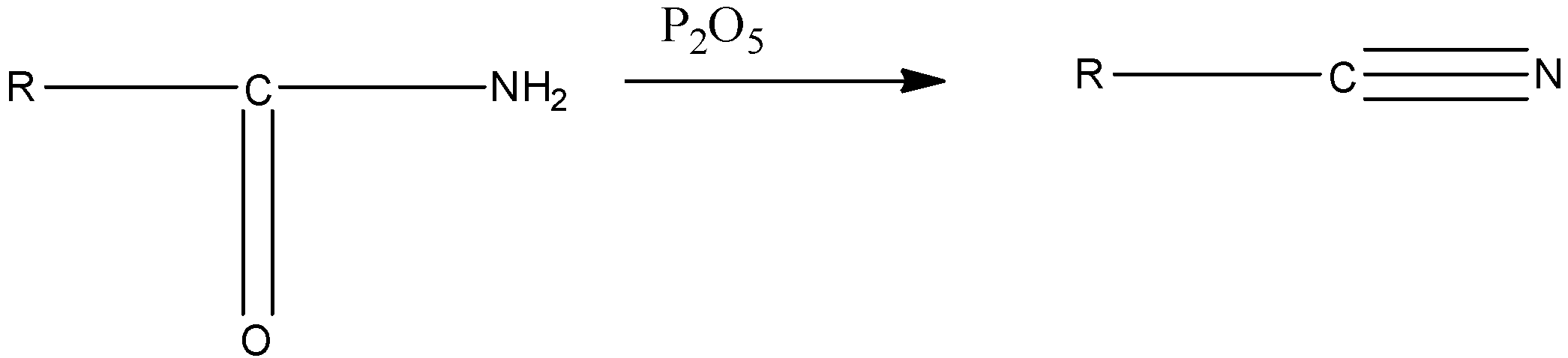
Option C, Phosphorous pentoxide($P_{ 2 }O_{ 5 }$) extracts water from many inorganic compounds including sulphuric acid, nitric acid, and several organic compounds. Therefore, It is used as a powerful dehydrating agent.
Example - It dehydrates amides to nitriles.
So, we can say that all of them are dehydrating agents.
So, the correct answer is “Option D”.
Note: The chemical formula of Phosphorous pentoxide is $P_{ 4 }O_{ 10 }$. However, it is named after its empirical formula, which is $P_{ 2 }O_{ 5 }$. This compound can also be used to convert certain mineral acids into their anhydrides. For example, $N_{ 2 }O_{ 5 }$ can be prepared from $HNO_3$ with the help of $P_{ 2 }O_{ 5 }$.
Complete step by step answer:
Let’s discuss all the options one by one to reach the correct answer -
Option A, Conc. $H_{ 2 }SO_{ 4 }$ (Concentrated sulphuric acid) has a greater affinity to water. Hence it can remove water molecules from other substances. That’s why it is called a dehydrating agent.
Example - Dehydration of an alcohol

Option B, $POCl_{ 3 }$ is a commonly used dehydrating agent in chemical laboratories. It is used as a dehydrating agent in the preparation of nitriles from primary amides.
Example - Dehydration of Benzamide

Option C, Phosphorous pentoxide($P_{ 2 }O_{ 5 }$) extracts water from many inorganic compounds including sulphuric acid, nitric acid, and several organic compounds. Therefore, It is used as a powerful dehydrating agent.
Example - It dehydrates amides to nitriles.

So, we can say that all of them are dehydrating agents.
So, the correct answer is “Option D”.
Note: The chemical formula of Phosphorous pentoxide is $P_{ 4 }O_{ 10 }$. However, it is named after its empirical formula, which is $P_{ 2 }O_{ 5 }$. This compound can also be used to convert certain mineral acids into their anhydrides. For example, $N_{ 2 }O_{ 5 }$ can be prepared from $HNO_3$ with the help of $P_{ 2 }O_{ 5 }$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




