
Which of the following can be used as an acid base indicator by a visually impaired student?
A) Methyl orange
B) Phenolphthalein
C) Olfactory indicator
D) Methyl red
Answer
537.6k+ views
Hint :An Olfactory indicator is the substance whose smell is different when it is added to acid and base respectively which helps the visually impaired students to detect the acid or base.
Complete Step By Step Answer:
An acid base indicator shows change of color from red to blue or blue to red. The change indicated by the indicator is as follows-
Red to blue color change indicates a base.
Blue to red color change indicates an acid.
Since visually impaired children cannot see the change they cannot identify the acid or base. So an olfactory indicator is used which smells different when it is added to acid or base. This particular odor helps students to detect the change of color.
The indicators methyl orange, phenolphthalein and methyl red do not give a particular odour during the change, they just show color change.
Hence the correct answer is ’C’.
Additional Information:
Some facts about the indicators-
The chemical formula of vanilla essence is ${{\text{C}}_8}{{\text{H}}_8}{{\text{O}}_3}$ . Its structure is-
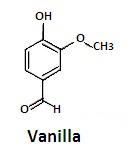
It is a phenolic aldehyde which has a fruity smell.
Methyl orange is a pH indicator with chemical formula${{\text{C}}_{14}}{{\text{H}}_{14}}{{\text{N}}_3}{\text{Na}}{{\text{O}}_3}{\text{S}}$. Its structure is-
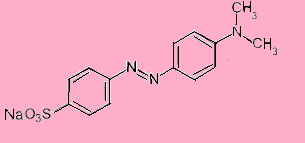
It gives red colour in acidic medium and yellow color in basic medium. It is mainly used in titration of acids.
3 Phenolphthalein is an acid-base indicator with chemical formula ${{\text{C}}_{20}}{{\text{H}}_{14}}{{\text{O}}_4}$ .Its structure is-
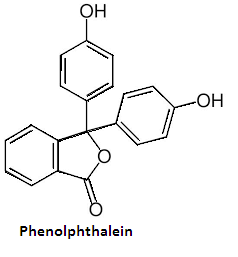
It gives pink color in the basic medium and turns colorless in the acidic medium.
Methyl Red is an azo dye with chemical formula${{\text{C}}_{15}}{{\text{H}}_{15}}{{\text{N}}_3}{{\text{O}}_2}$.Its structure is-
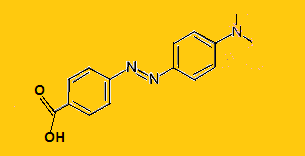
It gives red color in acidic medium and yellow in basic medium.
Note :
Vanilla essence is the best example of an olfactory indicator as it has a pleasant fruity smell. When it is added to an acidic solution, this characteristic smell remains but when it is added to a basic solution its smell vanishes. This gives the clue to the students whether it is acid or base. This process is called olfactory titration. Onion and clove oil can also be used as an olfactory indicator as they also have a characteristic smell. The basic solution generally destroys the particular odour of the olfactory indicator but acidic solution does not. This character of acid and base helps the visually impaired students while doing titration.
Complete Step By Step Answer:
An acid base indicator shows change of color from red to blue or blue to red. The change indicated by the indicator is as follows-
Red to blue color change indicates a base.
Blue to red color change indicates an acid.
Since visually impaired children cannot see the change they cannot identify the acid or base. So an olfactory indicator is used which smells different when it is added to acid or base. This particular odor helps students to detect the change of color.
The indicators methyl orange, phenolphthalein and methyl red do not give a particular odour during the change, they just show color change.
Hence the correct answer is ’C’.
Additional Information:
Some facts about the indicators-
The chemical formula of vanilla essence is ${{\text{C}}_8}{{\text{H}}_8}{{\text{O}}_3}$ . Its structure is-

It is a phenolic aldehyde which has a fruity smell.
Methyl orange is a pH indicator with chemical formula${{\text{C}}_{14}}{{\text{H}}_{14}}{{\text{N}}_3}{\text{Na}}{{\text{O}}_3}{\text{S}}$. Its structure is-

It gives red colour in acidic medium and yellow color in basic medium. It is mainly used in titration of acids.
3 Phenolphthalein is an acid-base indicator with chemical formula ${{\text{C}}_{20}}{{\text{H}}_{14}}{{\text{O}}_4}$ .Its structure is-

It gives pink color in the basic medium and turns colorless in the acidic medium.
Methyl Red is an azo dye with chemical formula${{\text{C}}_{15}}{{\text{H}}_{15}}{{\text{N}}_3}{{\text{O}}_2}$.Its structure is-

It gives red color in acidic medium and yellow in basic medium.
Note :
Vanilla essence is the best example of an olfactory indicator as it has a pleasant fruity smell. When it is added to an acidic solution, this characteristic smell remains but when it is added to a basic solution its smell vanishes. This gives the clue to the students whether it is acid or base. This process is called olfactory titration. Onion and clove oil can also be used as an olfactory indicator as they also have a characteristic smell. The basic solution generally destroys the particular odour of the olfactory indicator but acidic solution does not. This character of acid and base helps the visually impaired students while doing titration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




