
Which of the following can be measured by the Oswald-Walker dynamic method?
A.Relative lowering of vapour pressure
B.Lowering of vapour pressure
C.Vapour pressure of solvent
D.All of these.
Answer
589.5k+ views
Hint: Oswald-Walker Dynamic method is useful in measuring some particular colligative property. Colligative properties are those properties of solutions which depend on the ratio of the number of solvent molecules in a solution and not on the nature of the chemical species present.
Complete answer:
First let us discuss about that is Oswald-Walker Dynamic method, this method is based on the principle that when we successively pass dry air through a series of containers containing solution and pure solvent respectively then the air which is passed get saturated with the solvent vapours here the when this happen an equal amount of weight loss in solution and solvent take place which is filled in the containers.
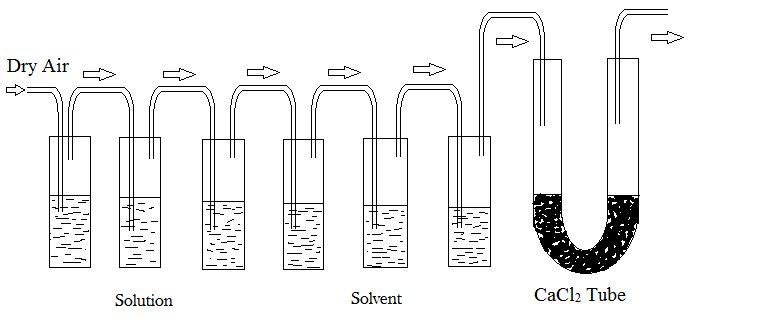
As Relative lowering of vapour pressure is the vapour above the solution consist of only solvent which is the pure liquid where the solute molecules are non-volatile, after adding the solute the vapour pressure of the solution is observed to be lower than the pure liquid at a given temperature which we know here as Relative lowering of vapour pressure and there are some methods to find it one of which is the Oswald-Walker Dynamic method so we can say that relative lowering of vapour pressure is the answer.
Note:
The point we need to know is that the relative lowering of vapour pressure is the ratio of vapour pressure lowering of solvent from solution to the vapour pressure of pure solvent which is used in the Oswald-Walker Dynamic method to calculate the relative lowering of vapour pressure.
One more point is vapour pressure is one of the colligative properties so as relative lowering where decrease in vapour pressure depends on the amount of non-volatile solute which is added to the solution irrespective of its nature hence, we can conclude that it is also one of the colligative properties.
Complete answer:
First let us discuss about that is Oswald-Walker Dynamic method, this method is based on the principle that when we successively pass dry air through a series of containers containing solution and pure solvent respectively then the air which is passed get saturated with the solvent vapours here the when this happen an equal amount of weight loss in solution and solvent take place which is filled in the containers.

As Relative lowering of vapour pressure is the vapour above the solution consist of only solvent which is the pure liquid where the solute molecules are non-volatile, after adding the solute the vapour pressure of the solution is observed to be lower than the pure liquid at a given temperature which we know here as Relative lowering of vapour pressure and there are some methods to find it one of which is the Oswald-Walker Dynamic method so we can say that relative lowering of vapour pressure is the answer.
Note:
The point we need to know is that the relative lowering of vapour pressure is the ratio of vapour pressure lowering of solvent from solution to the vapour pressure of pure solvent which is used in the Oswald-Walker Dynamic method to calculate the relative lowering of vapour pressure.
One more point is vapour pressure is one of the colligative properties so as relative lowering where decrease in vapour pressure depends on the amount of non-volatile solute which is added to the solution irrespective of its nature hence, we can conclude that it is also one of the colligative properties.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




