
Which of the following can act as Lewis acid?
(This question has multiple correct options)
(A) $Si{{F}_{4}}$
(B) $SnC{{l}_{4}}$
(C) $CC{{l}_{4}}$
(D) $S{{F}_{4}}$
Answer
593.1k+ views
Hint: Lewis acid is a chemical species that contains an empty orbital which is capable of accepting an electron pair. So, find the compounds that can accept an electron pair and make a covalent coordinate bond with a lewis base.
Complete step by step answer:
At first we should clear the concept of Lewis acid and bases in our mind. Let us take a reaction first, which is represented below:
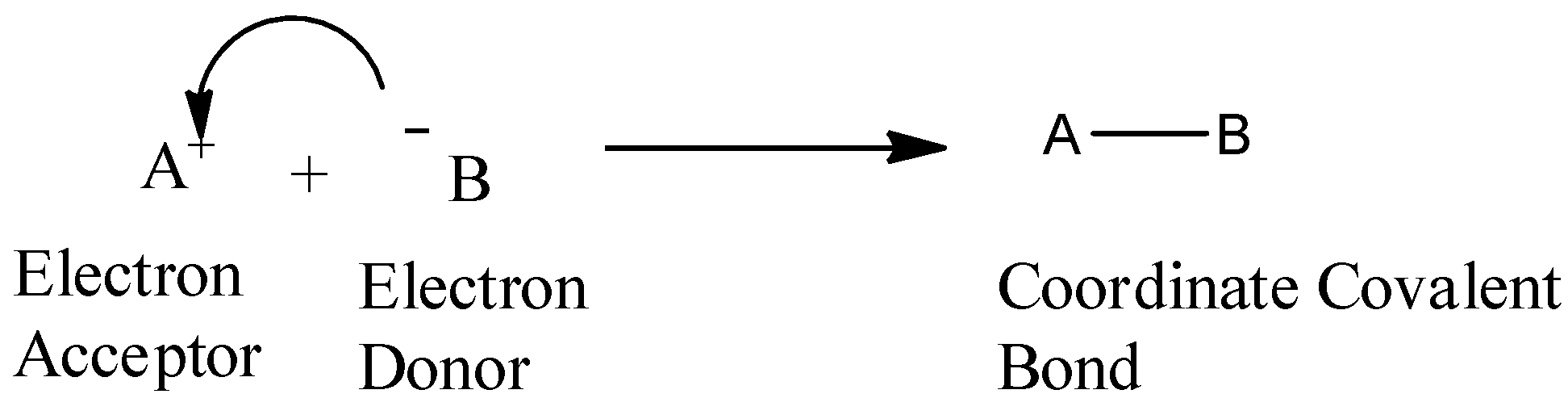
- As shown in the above reaction, we should note that reaction of a Lewis acid and a Lewis base will produce a coordinate covalent bond. We should note that a coordinate covalent bond is just a type of covalent bond in which one reactant gives its electron pair to another reactant. In the above reaction, Lewis base donates its electrons to the Lewis acid. We should remember that Lewis Acid, is a species that accepts an electron pair (i.e., an electrophile) and will have vacant orbitals. On the other hand we can say that Lewis Base is a species that donates an electron pair (i.e., a nucleophile) and will have lone-pair electrons.
- Now, we know about the concept of Lewis acid and Lewis base. We will now check the options to check whether there is any Lewis acid in options or not. We should know that molecules where the central atom can have more than 8 valence shell electrons can be electron acceptors due to the presence of empty d-orbitals, and thus, are classified as Lewis acids.
- Let us check the first option. We know that in silicon tetrafluoride, the central atom is Silicon. So, electronic configuration is $[Ne]3{{s}^{2}}3{{p}^{2}}$. We know that it has an empty 3d orbital. So, it will act as Lewis acid.
- Let us take the second option of tin tetrachloride. We should now write the electronic configuration of tin. The electronic configuration of Tin is \[\left[ Kr \right]4{{d}^{10}}5{{s}^{2}}5{{p}^{2}}\]. So, it has an empty d-orbital. Hence we can say that it will also act as Lewis acid.
- Let us take the third option of carbon tetrachloride. Electronic configuration of carbon is \[\left[ He \right]\text{ }2{{s}^{2}}\text{ }2{{p}^{2}}\]. We note that it doesn’t have d orbital. So, it will not act as Lewis acid.
- The fourth option is Sulphur tetrafluoride. Electronic configuration of sulphur is \[\left[ Ne \right]3{{s}^{2}}\text{ }3{{p}^{4}}\]. It has an empty d orbital, so it will accept electrons. That’s why it will act as Lewis acid.
Hence option A, B and D will act as Lewis acid.
Note:
Let us know about Lewis Bases. We should note that Lewis Bases donate an electron pair. Lewis Bases are Nucleophile. It means that they attack a positive charge with their lone pair. We should know that an atom, ion, or molecule with a lone-pair of electrons can thus be a Lewis base. Each of the following anions can give up their electrons to an acid, for an example:
\[O{{H}^{-}},~C{{N}^{-}},~C{{H}_{3}}CO{{O}^{-}},~N{{H}_{3}},~{{H}_{2}}O,~CO\]
Complete step by step answer:
At first we should clear the concept of Lewis acid and bases in our mind. Let us take a reaction first, which is represented below:

- As shown in the above reaction, we should note that reaction of a Lewis acid and a Lewis base will produce a coordinate covalent bond. We should note that a coordinate covalent bond is just a type of covalent bond in which one reactant gives its electron pair to another reactant. In the above reaction, Lewis base donates its electrons to the Lewis acid. We should remember that Lewis Acid, is a species that accepts an electron pair (i.e., an electrophile) and will have vacant orbitals. On the other hand we can say that Lewis Base is a species that donates an electron pair (i.e., a nucleophile) and will have lone-pair electrons.
- Now, we know about the concept of Lewis acid and Lewis base. We will now check the options to check whether there is any Lewis acid in options or not. We should know that molecules where the central atom can have more than 8 valence shell electrons can be electron acceptors due to the presence of empty d-orbitals, and thus, are classified as Lewis acids.
- Let us check the first option. We know that in silicon tetrafluoride, the central atom is Silicon. So, electronic configuration is $[Ne]3{{s}^{2}}3{{p}^{2}}$. We know that it has an empty 3d orbital. So, it will act as Lewis acid.
- Let us take the second option of tin tetrachloride. We should now write the electronic configuration of tin. The electronic configuration of Tin is \[\left[ Kr \right]4{{d}^{10}}5{{s}^{2}}5{{p}^{2}}\]. So, it has an empty d-orbital. Hence we can say that it will also act as Lewis acid.
- Let us take the third option of carbon tetrachloride. Electronic configuration of carbon is \[\left[ He \right]\text{ }2{{s}^{2}}\text{ }2{{p}^{2}}\]. We note that it doesn’t have d orbital. So, it will not act as Lewis acid.
- The fourth option is Sulphur tetrafluoride. Electronic configuration of sulphur is \[\left[ Ne \right]3{{s}^{2}}\text{ }3{{p}^{4}}\]. It has an empty d orbital, so it will accept electrons. That’s why it will act as Lewis acid.
Hence option A, B and D will act as Lewis acid.
Note:
Let us know about Lewis Bases. We should note that Lewis Bases donate an electron pair. Lewis Bases are Nucleophile. It means that they attack a positive charge with their lone pair. We should know that an atom, ion, or molecule with a lone-pair of electrons can thus be a Lewis base. Each of the following anions can give up their electrons to an acid, for an example:
\[O{{H}^{-}},~C{{N}^{-}},~C{{H}_{3}}CO{{O}^{-}},~N{{H}_{3}},~{{H}_{2}}O,~CO\]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




