
Which of the following best describes what happens in the first step in the mechanism of the hydrogen -deuterium exchange reaction shown?
$C{H_3}C \equiv CD\xrightarrow[{N{H_3}}]{{NaN{H_2}}}C{H_3}C \equiv CH$
A.
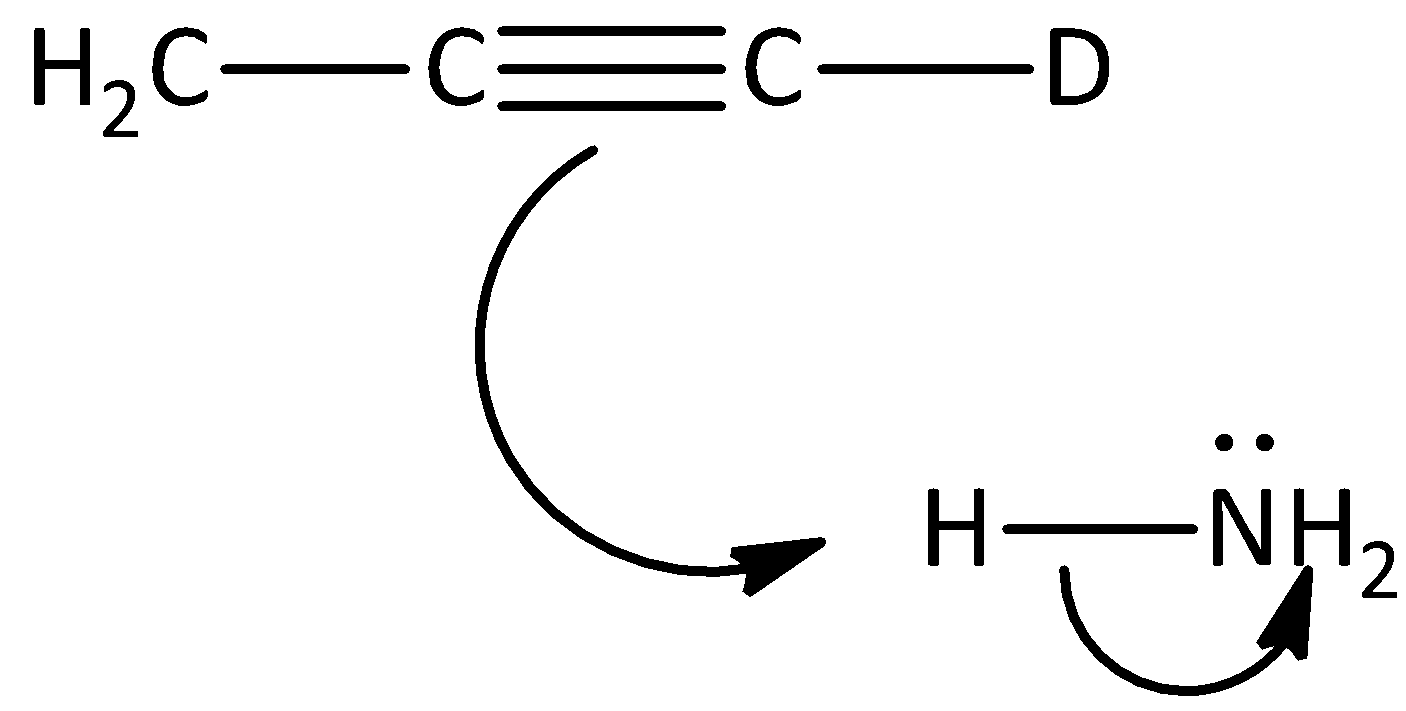
B.

C.
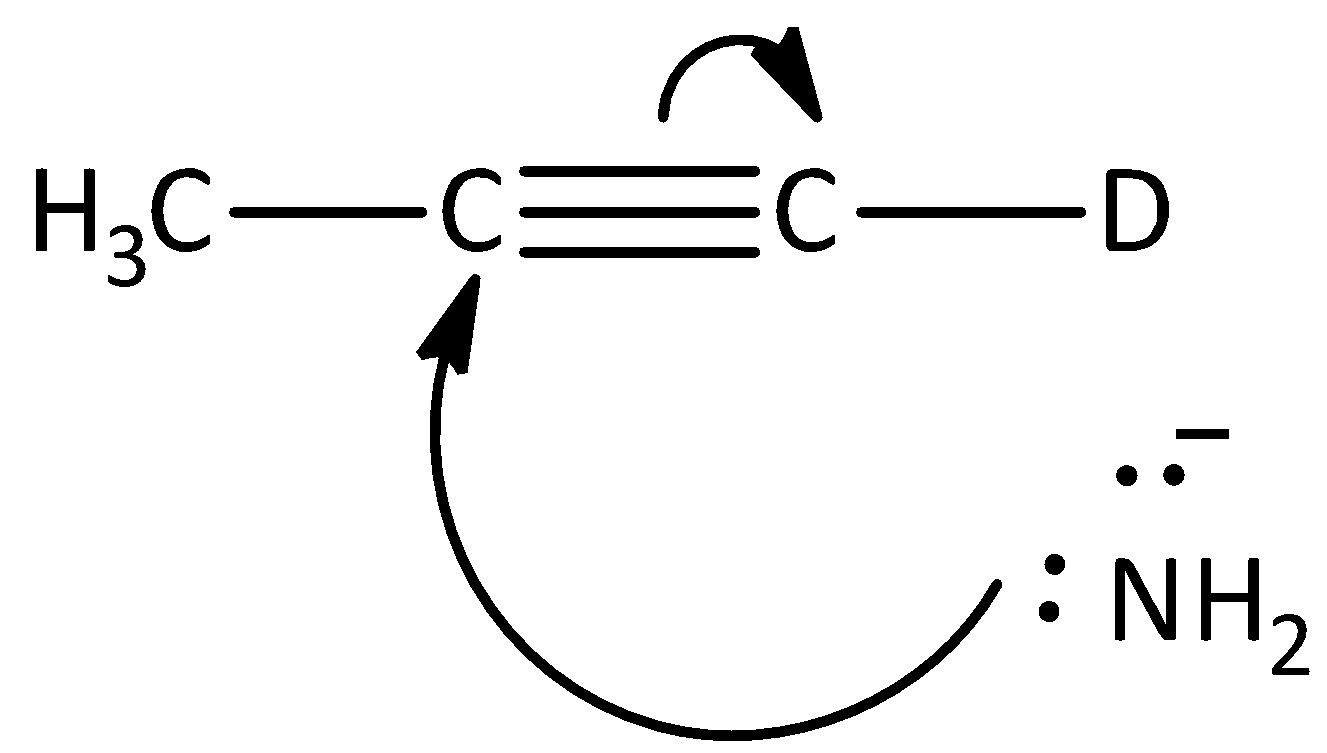
D.
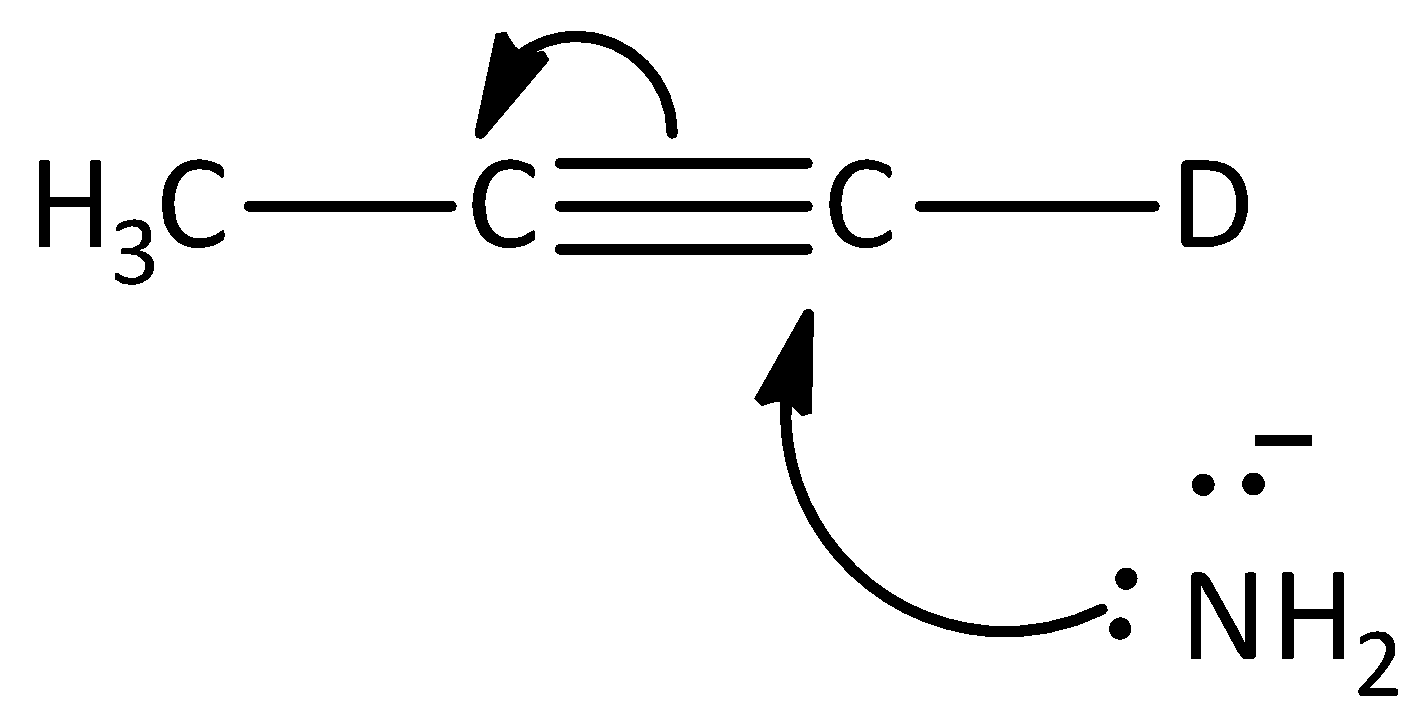




Answer
497.7k+ views
Hint: We need to know that the hydrogen – deuterium exchange reaction is also known as $H - D$ exchange. The hydrogen – deuterium exchange reaction is a chemical reaction, here the covalently attached hydrogen atom is replaced by using deuterium atom or the deuterium atom is replaced by covalently bonded hydrogen atom and there occurs perdeuteration. The deuterium is a chemical compound having the symbol, \[{D_2}O\] and the deuterium is also known as heavy water. The heavy water is used in many industries like NMR spectroscopy, nuclear reactors, etc.
Complete answer:
The hydrogen-deuterium exchange reaction takes place in the presence of sodamide and ammonia. Let’s see the reaction;
\[C{H_3}C \equiv CD\xrightarrow[{N{H_3}}]{{NaN{H_2}}}C{H_3}C \equiv CH\]
Here, \[C{H_3}C \equiv CD\] is reacted with ammonia and sodamide, and the deuterium is replaced by covalently bonded hydrogen atoms present in the \[C{H_3}C \equiv CH\]. Therefore, the stable conjugate base part will attack the hydrogen present in the ammonia and there is a formation of \[C{H_3}C \equiv CH\]. Hence, we can say that, the first step of mechanism in the mechanism of the hydrogen -deuterium exchange reaction is,
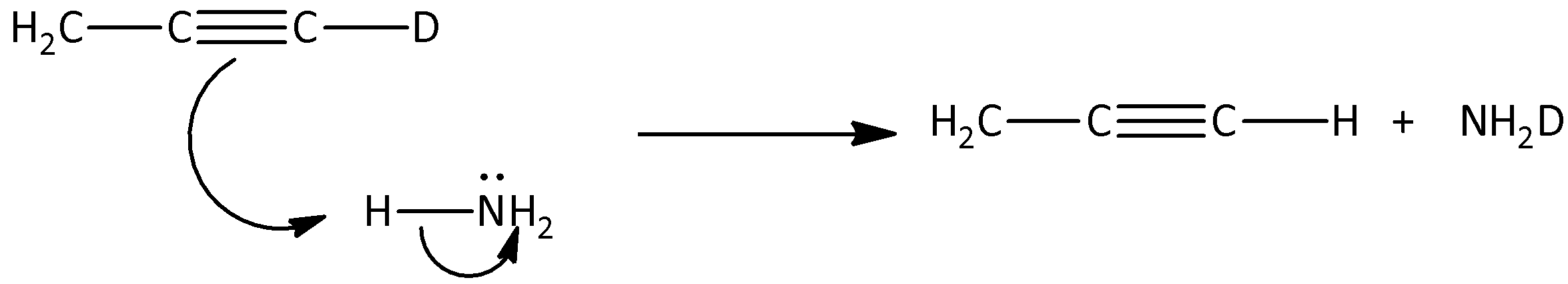
Hence, option (A) is correct.
The given reaction is not the first step of the mechanism in the hydrogen -deuterium exchange reaction. Hence, option (B) is incorrect.
The given reaction is not the first step of the mechanism in the hydrogen -deuterium exchange reaction. Hence, option (C) is incorrect.
The given reaction is not the first step of the mechanism in the hydrogen -deuterium exchange reaction. Hence, option (D) is incorrect.
Hence, option (A) is correct.
Note:
We have to remember that in hydrogen -deuterium exchange reaction, the covalently bonded alpha-hydrogen atom is replaced by using deuterium in the presence of sodamide and ammonia. The hydrogen is replaced because of its acidic nature of the alpha-hydrogen atom. And this reaction can be accelerated by adding a base or an acid. At the end of the reaction, the complete alpha – hydrogen is exchanged by the deuterium atom.
Complete answer:
The hydrogen-deuterium exchange reaction takes place in the presence of sodamide and ammonia. Let’s see the reaction;
\[C{H_3}C \equiv CD\xrightarrow[{N{H_3}}]{{NaN{H_2}}}C{H_3}C \equiv CH\]
Here, \[C{H_3}C \equiv CD\] is reacted with ammonia and sodamide, and the deuterium is replaced by covalently bonded hydrogen atoms present in the \[C{H_3}C \equiv CH\]. Therefore, the stable conjugate base part will attack the hydrogen present in the ammonia and there is a formation of \[C{H_3}C \equiv CH\]. Hence, we can say that, the first step of mechanism in the mechanism of the hydrogen -deuterium exchange reaction is,

Hence, option (A) is correct.
The given reaction is not the first step of the mechanism in the hydrogen -deuterium exchange reaction. Hence, option (B) is incorrect.
The given reaction is not the first step of the mechanism in the hydrogen -deuterium exchange reaction. Hence, option (C) is incorrect.
The given reaction is not the first step of the mechanism in the hydrogen -deuterium exchange reaction. Hence, option (D) is incorrect.
Hence, option (A) is correct.
Note:
We have to remember that in hydrogen -deuterium exchange reaction, the covalently bonded alpha-hydrogen atom is replaced by using deuterium in the presence of sodamide and ammonia. The hydrogen is replaced because of its acidic nature of the alpha-hydrogen atom. And this reaction can be accelerated by adding a base or an acid. At the end of the reaction, the complete alpha – hydrogen is exchanged by the deuterium atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




