
Which of the following basic compounds is present in coal tar?
A . Quinoline
B. Benzene
C. Toluene
D. Phenol
Answer
571.8k+ views
Hint:Coal tar is by products of coal pyrolysis to produce coke/natural gas. They are viscous liquid or semi solids that are black or dark brown in colour and with naphthalene like odour. The liquid mixture contains various kinds of useful compounds.
Coal tar are complex combinations of polycyclic aromatic hydrocarbons , phenols, heterocyclic oxygen, sulphur and nitrogen compounds. Coal tar contains an estimated 10,000 chemical compounds of which around 400 have been identified.
The 55% of identified components in coal tar is the source of aromatic compounds. The composition of coal tar varies greatly depending on the type of coal and the coking process and temperature.
Complete answer:
Generally coal utilization techniques are divided into combustion, pyrolysis, liquefaction, gasification. Where pyrolysis technique is a technique of heating coal in high temperature ( $\mathop {{\text{500 - 1000}}}\nolimits^{\text{o}} {\text{C}}$) without contact with air , which produce coke (coal with high calorific combustion ),gas and tar.
When coal pyrolysis and distillation are conducted by heating without contact with air coal will be converted into solid liquid and gas.
Coal tar contains more than 348 types of chemical compounds which are very valuable , they are aromatic compounds( such as benzene , toluene, xylene ,naphthalene , anthracene etc..)heterocyclic nitrogen compounds ( pyridine, quinoline , isoquinoline , indole etc) and oxy heterocyclic compounds such as dibenzofuran etc . which all can be used as raw materials or intermediate materials in various chemical industries.
Since coal tar consists of all the above compounds, benzene and toluene are neutral compounds , phenol is slightly acidic compound and quinoline is the basic compound.
Structure of quinoline is given by
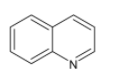
Quinoline is a heterocyclic aromatic compound consisting of a double ring structure that contains a benzene ring fused to pyridine at two adjacent carbon atoms.it is a weak tertiary base.
So here the answer is option A.
Note:
Quinoline i is also known as Benzo[b]pyridine or chinoline.it is an heterocyclic aromatic compound. It has a slightly weaker base . It reacts with acids to yield salts which are sparingly soluble in water.
Coal tar are complex combinations of polycyclic aromatic hydrocarbons , phenols, heterocyclic oxygen, sulphur and nitrogen compounds. Coal tar contains an estimated 10,000 chemical compounds of which around 400 have been identified.
The 55% of identified components in coal tar is the source of aromatic compounds. The composition of coal tar varies greatly depending on the type of coal and the coking process and temperature.
Complete answer:
Generally coal utilization techniques are divided into combustion, pyrolysis, liquefaction, gasification. Where pyrolysis technique is a technique of heating coal in high temperature ( $\mathop {{\text{500 - 1000}}}\nolimits^{\text{o}} {\text{C}}$) without contact with air , which produce coke (coal with high calorific combustion ),gas and tar.
When coal pyrolysis and distillation are conducted by heating without contact with air coal will be converted into solid liquid and gas.
Coal tar contains more than 348 types of chemical compounds which are very valuable , they are aromatic compounds( such as benzene , toluene, xylene ,naphthalene , anthracene etc..)heterocyclic nitrogen compounds ( pyridine, quinoline , isoquinoline , indole etc) and oxy heterocyclic compounds such as dibenzofuran etc . which all can be used as raw materials or intermediate materials in various chemical industries.
Since coal tar consists of all the above compounds, benzene and toluene are neutral compounds , phenol is slightly acidic compound and quinoline is the basic compound.
Structure of quinoline is given by

Quinoline is a heterocyclic aromatic compound consisting of a double ring structure that contains a benzene ring fused to pyridine at two adjacent carbon atoms.it is a weak tertiary base.
So here the answer is option A.
Note:
Quinoline i is also known as Benzo[b]pyridine or chinoline.it is an heterocyclic aromatic compound. It has a slightly weaker base . It reacts with acids to yield salts which are sparingly soluble in water.
Recently Updated Pages
Master Class 8 Social Science: Engaging Questions & Answers for Success

Master Class 8 English: Engaging Questions & Answers for Success

Class 8 Question and Answer - Your Ultimate Solutions Guide

Master Class 8 Maths: Engaging Questions & Answers for Success

Master Class 8 Science: Engaging Questions & Answers for Success

Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Trending doubts
What is BLO What is the full form of BLO class 8 social science CBSE

Citizens of India can vote at the age of A 18 years class 8 social science CBSE

Full form of STD, ISD and PCO

Advantages and disadvantages of science

Right to vote is a AFundamental Right BFundamental class 8 social science CBSE

What are the 12 elements of nature class 8 chemistry CBSE




