
Which of the following aryl amines undergoes diazotization most readily?
(A)
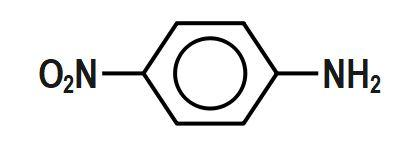
(B)
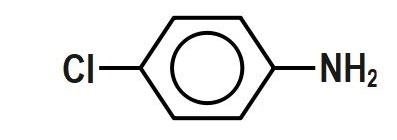
(C)
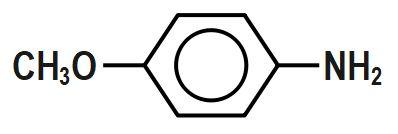
(D)
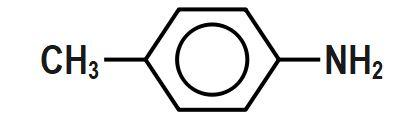




Answer
514.2k+ views
Hint :We know that the process of producing diazonium salts or diazonium compounds is called diazotization or diazoniation or diazotation. It was first given by Peter Griess. Thus, diazotization is the process used in the formation of diazonium salts through aromatic amines.
Complete Step By Step Answer:
The answer for this question is based on the concept of reactions of organic chemistry where the diazotization involves treatment of primary aromatic amines with nitrous acid and carbylamine reaction involves reaction of primary amine with chloroform and base. Aromatic amine reacts with nitrous acid and mineral acid to form diazonium salt and produces water as a side product. This reaction is known as Diazotization Reaction. The reaction can be represented in word reaction form. The concepts of several named reactions and also some of the basic reactions like addition reactions, substitution reactions, elimination reactions, halogenations, cyanation, etc. are familiar to us from the chapters of organic chemistry.
Diazotization is the reaction in which aromatic amines that are the primary amines when treated with nitrous acid and mineral acid produce diazonium salt with the release of water as a by-product. From the given aryl amine, the one that undergoes diazotization most readily, in the first option, the withdrawing nature of the NO₂ group leads to diazotization most readily. Diazotization can be defined as the process of conversion of primary aromatic amines into its diazonium salt. These salts can then undergo coupling reactions to form azo dyes. But as option C is p-methoxyaniline is a fast diazotizing group because it contains an excellent electron donating group which facilitates the formation of diazonium salt.
Therefore, the correct answer is option C.
Note :
Remember that the diazotization reactions are to be carried out at lower temperatures because if we employ higher temperatures then the salts form others by products and thus result in the formation of phenol by the reaction with water at higher temperature.
Complete Step By Step Answer:
The answer for this question is based on the concept of reactions of organic chemistry where the diazotization involves treatment of primary aromatic amines with nitrous acid and carbylamine reaction involves reaction of primary amine with chloroform and base. Aromatic amine reacts with nitrous acid and mineral acid to form diazonium salt and produces water as a side product. This reaction is known as Diazotization Reaction. The reaction can be represented in word reaction form. The concepts of several named reactions and also some of the basic reactions like addition reactions, substitution reactions, elimination reactions, halogenations, cyanation, etc. are familiar to us from the chapters of organic chemistry.
Diazotization is the reaction in which aromatic amines that are the primary amines when treated with nitrous acid and mineral acid produce diazonium salt with the release of water as a by-product. From the given aryl amine, the one that undergoes diazotization most readily, in the first option, the withdrawing nature of the NO₂ group leads to diazotization most readily. Diazotization can be defined as the process of conversion of primary aromatic amines into its diazonium salt. These salts can then undergo coupling reactions to form azo dyes. But as option C is p-methoxyaniline is a fast diazotizing group because it contains an excellent electron donating group which facilitates the formation of diazonium salt.
Therefore, the correct answer is option C.
Note :
Remember that the diazotization reactions are to be carried out at lower temperatures because if we employ higher temperatures then the salts form others by products and thus result in the formation of phenol by the reaction with water at higher temperature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




