
Which of the following are resolvable:
A.
B.
C.
D.




Answer
573k+ views
Hint: To answer this question, you should recall the concept of optical isomerism. To identify resolvable and non-resolvable isomers you need to know about the presence of a chiral carbon and should not contain any symmetry.
Complete step by step solution:
For any chemical entity to be optically active it should have a chiral carbon and should not contain any symmetry. Resolving means conversion of an optically inactive compound to the optically active compound. Make sure you remember that meso compounds will never be resolved.
Let us analyse the options systematically:
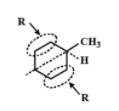
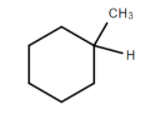
Does not have an asymmetrical carbon atom because two R groups are the same.
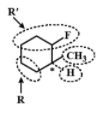
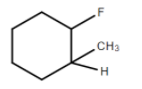
It is optically active since it has an asymmetrical C atom. Four different groups are \[R,\;R\prime ,C{H_3}{\text{ and}}\;H\]
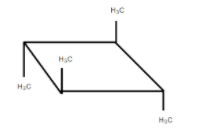
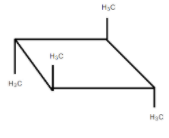
It is optically inactive due to the presence of diagonal planes of symmetry and the alternating axis of symmetry.
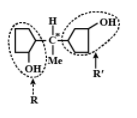
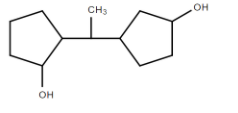
It is optically active since it has asymmetrical carbon atoms. Four different groups are \[H,Me,R,{\text{ and}}R\prime \]
Hence,options B and D are correct.
Note:
Meso compounds will never be resolved. In general, a meso compound should contain two or more identical substituted stereocenters. This molecule also has an internal symmetry plane that divides the compound in half. These two halves reflect each other by the internal mirror. This results in the stereochemistry being cancelled out by the two stereocenters hence resulting in an optically inactive mixture. If A is a meso compound, it should have two or more stereocenters, an internal plane, and the stereochemistry should be R and S:
Look for an internal plane, or internal mirror, that lies in between the compound.
R cancels S out in a meso compound with two stereo centres making it optically inactive.
Complete step by step solution:
For any chemical entity to be optically active it should have a chiral carbon and should not contain any symmetry. Resolving means conversion of an optically inactive compound to the optically active compound. Make sure you remember that meso compounds will never be resolved.
Let us analyse the options systematically:

Does not have an asymmetrical carbon atom because two R groups are the same.

It is optically active since it has an asymmetrical C atom. Four different groups are \[R,\;R\prime ,C{H_3}{\text{ and}}\;H\]

It is optically inactive due to the presence of diagonal planes of symmetry and the alternating axis of symmetry.

It is optically active since it has asymmetrical carbon atoms. Four different groups are \[H,Me,R,{\text{ and}}R\prime \]
Hence,options B and D are correct.
Note:
Meso compounds will never be resolved. In general, a meso compound should contain two or more identical substituted stereocenters. This molecule also has an internal symmetry plane that divides the compound in half. These two halves reflect each other by the internal mirror. This results in the stereochemistry being cancelled out by the two stereocenters hence resulting in an optically inactive mixture. If A is a meso compound, it should have two or more stereocenters, an internal plane, and the stereochemistry should be R and S:
Look for an internal plane, or internal mirror, that lies in between the compound.
R cancels S out in a meso compound with two stereo centres making it optically inactive.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




