
Which of the following alkyl halides undergoes ${S_N}1$ reaction the fastest?
A. methyl chloride
B. ethyl chloride
C. isobutyl chloride
D. tert-butyl chloride
Answer
585.9k+ views
Hint: Alkyl halides are a class of organic compounds in which an alkyl group is attached to a halogen atom. The alkyl group is made of carbon and hydrogen atoms. Examples of alkyl groups are methyl, ethyl, isobutyl and tert-butyl. \[{S_N}1\] reaction is a type of substitution whose rate is dependent on the formation of one species i.e. carbocation.
Complete step by step answer: The question is related to substitution reaction of aliphatic compounds. In substitution reaction a leaving group is present in the substrate which is substituted by another group. The substitution can occur stepwise or in one step. There are various types of substitution reactions like \[{S_N}1\], \[{S_N}2\], \[{S_N}i\] etc. Here we will try to understand the \[{S_N}1\] reaction mechanism.
In Sn1 type of substitution, the reaction goes through a two step process. The first step is the formation of the corresponding carbocation and the second step is the attack of the nucleophile on the carbocation to produce the product. The following diagram is shown for \[{S_N}1\] type of substitution.
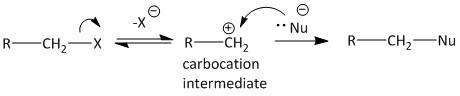
The formation of carbocation is the rate determining step for this type of reaction. The stability of the carbocation is the essential criteria for the reaction to proceed faster.
For the given list of compounds, methyl chloride, ethyl chloride and isobutyl chloride will generate \[1^\circ \] carbocation by loss of chloride group while tert butyl chloride will generate \[3^\circ \] carbocation. As the stability of carbocation is\[3^\circ > 2^\circ > 1^\circ \], the tert butyl carbocation will be most stable. This is due to the electron donation power of methyl group also called inductive effect which satisfies the electron deficiency of carbocation.
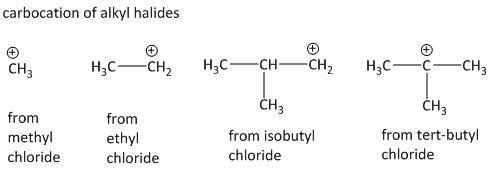
Thus tert-butyl chloride having the most stable carbocation will undergo the fastest \[{S_N}1\] reaction.
Note: The substitution reaction is not limited to aliphatic compounds. Aromatic compounds undergo faster \[{S_N}1\] reaction than aliphatic compounds. In aromatic compounds the intermediate carbocation is resonance stabilized. Resonance effect is more powerful than inductive effect due to high stabilization energy.
Complete step by step answer: The question is related to substitution reaction of aliphatic compounds. In substitution reaction a leaving group is present in the substrate which is substituted by another group. The substitution can occur stepwise or in one step. There are various types of substitution reactions like \[{S_N}1\], \[{S_N}2\], \[{S_N}i\] etc. Here we will try to understand the \[{S_N}1\] reaction mechanism.
In Sn1 type of substitution, the reaction goes through a two step process. The first step is the formation of the corresponding carbocation and the second step is the attack of the nucleophile on the carbocation to produce the product. The following diagram is shown for \[{S_N}1\] type of substitution.

The formation of carbocation is the rate determining step for this type of reaction. The stability of the carbocation is the essential criteria for the reaction to proceed faster.
For the given list of compounds, methyl chloride, ethyl chloride and isobutyl chloride will generate \[1^\circ \] carbocation by loss of chloride group while tert butyl chloride will generate \[3^\circ \] carbocation. As the stability of carbocation is\[3^\circ > 2^\circ > 1^\circ \], the tert butyl carbocation will be most stable. This is due to the electron donation power of methyl group also called inductive effect which satisfies the electron deficiency of carbocation.

Thus tert-butyl chloride having the most stable carbocation will undergo the fastest \[{S_N}1\] reaction.
Note: The substitution reaction is not limited to aliphatic compounds. Aromatic compounds undergo faster \[{S_N}1\] reaction than aliphatic compounds. In aromatic compounds the intermediate carbocation is resonance stabilized. Resonance effect is more powerful than inductive effect due to high stabilization energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




