
Which of the following alcohols cannot be prepared from hydration of an alkene?
A.
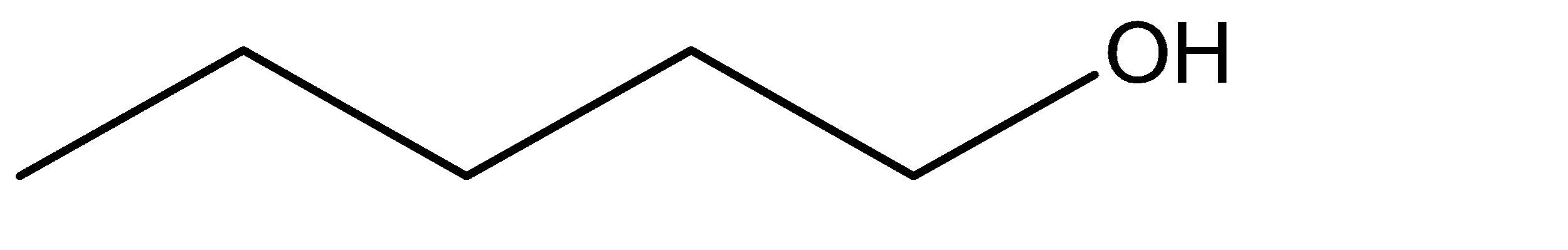
B.
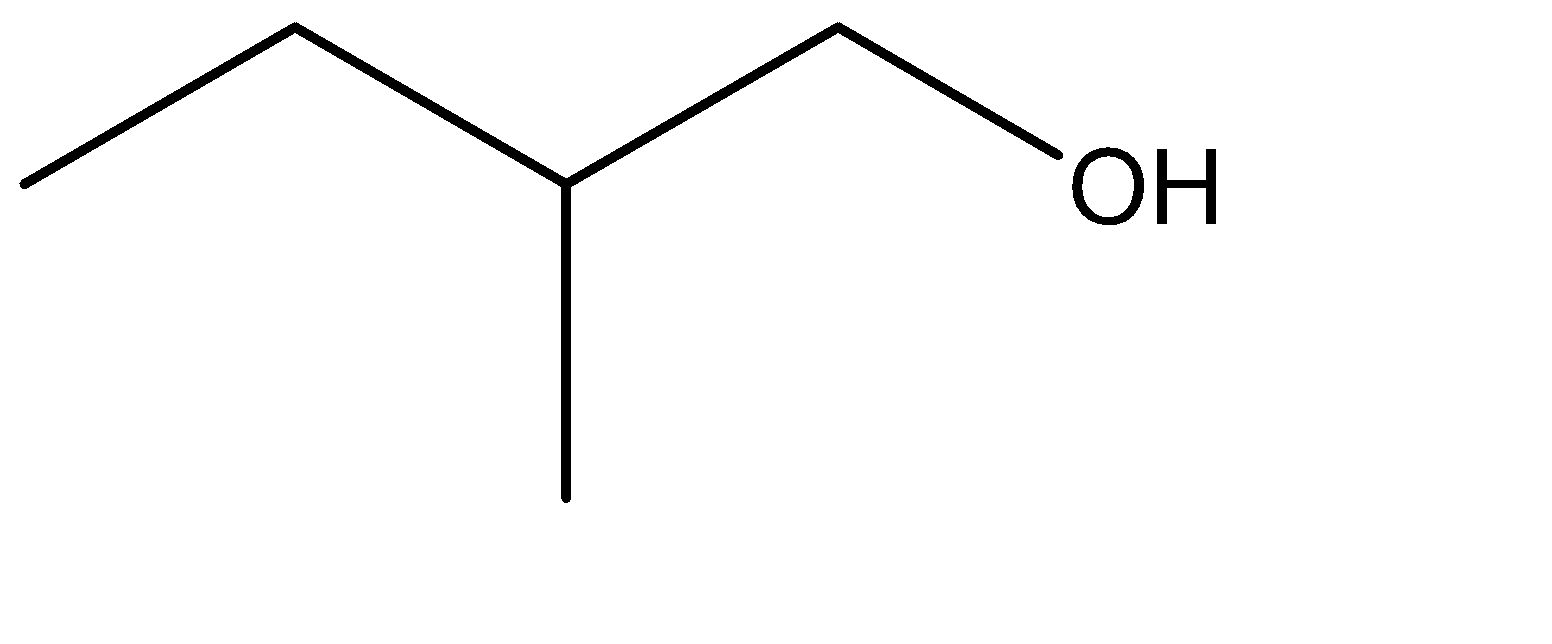
C.
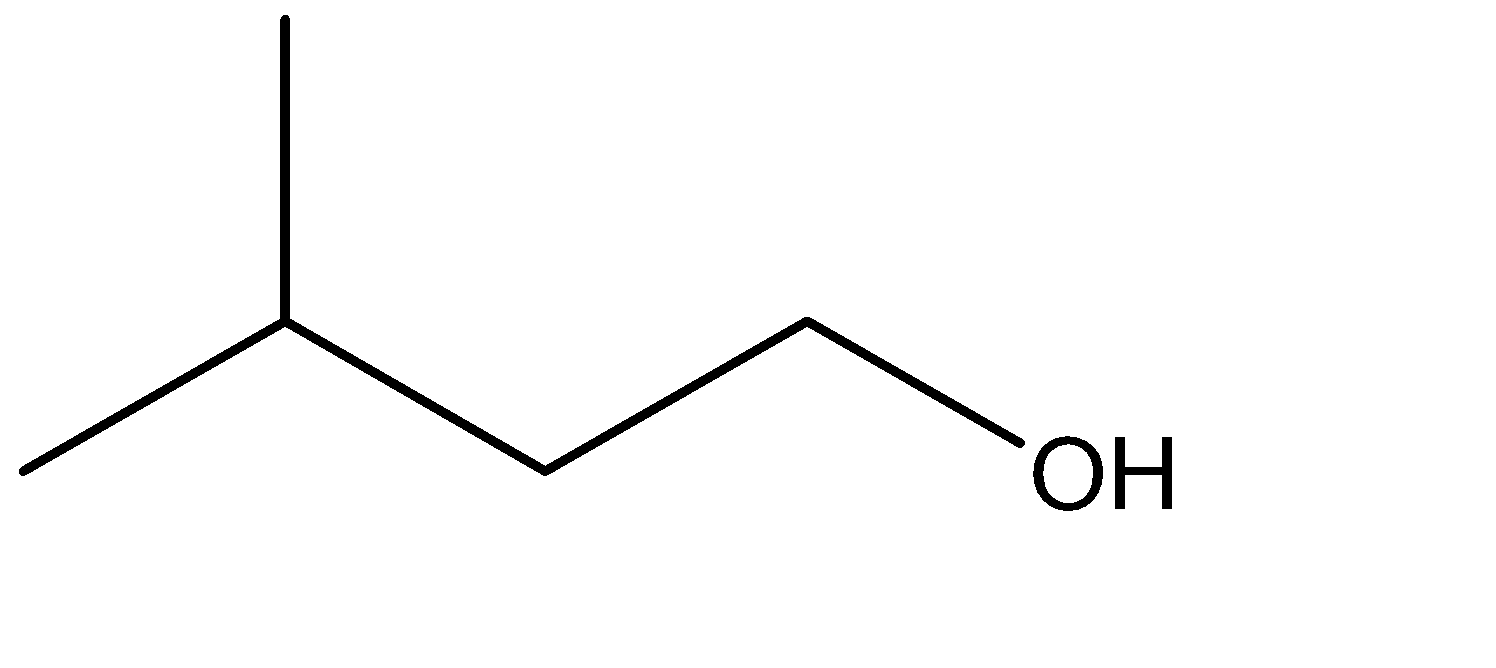
D.
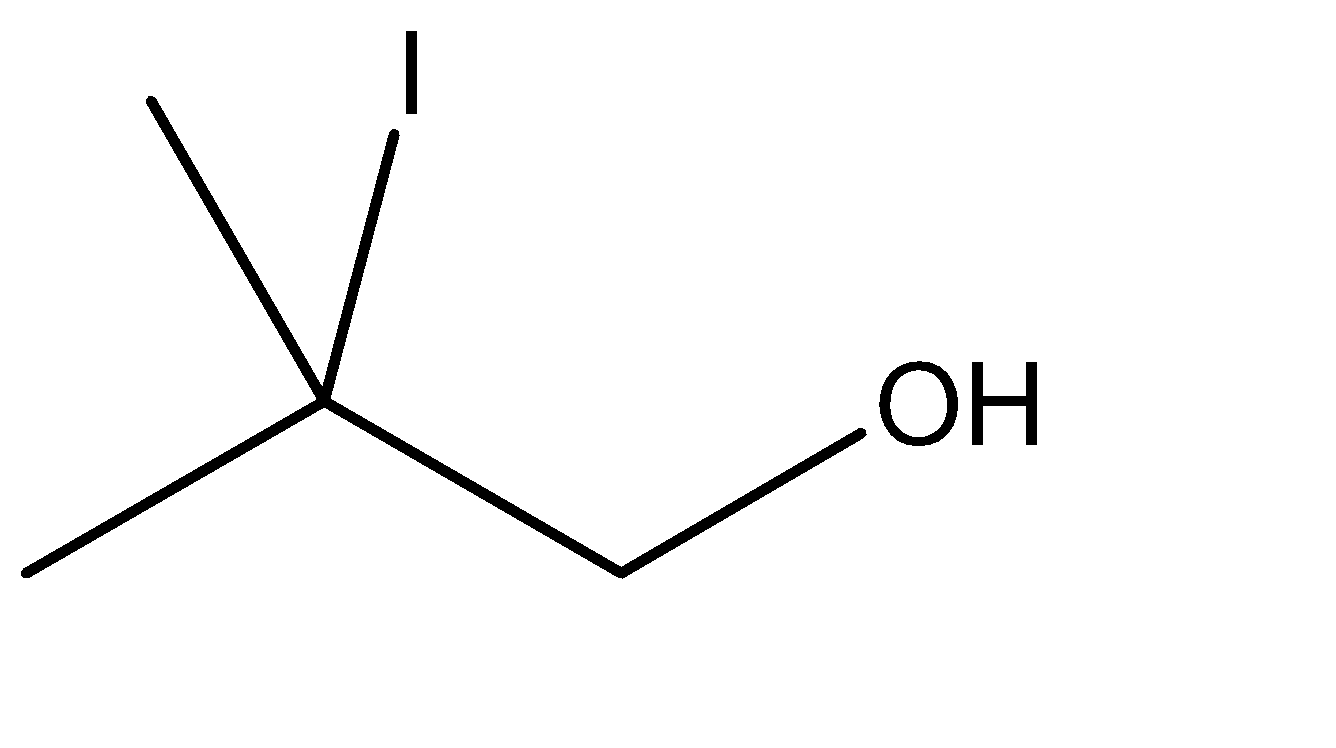




Answer
582.6k+ views
Hint: Hydration of alkenes can be understood as the conversion of the alkene to alkane. This means that the pi bond in alkenes is broken to form a single sigma bond, with the help of a water molecule in most cases. This also results in formation of an alcoholic functional group at the carbon atom where the bond is broken. Different types of alkenes require different conditions for hydration.
Complete Step-by-Step Answer:
Let us discuss the options one by one to discuss each case separately.
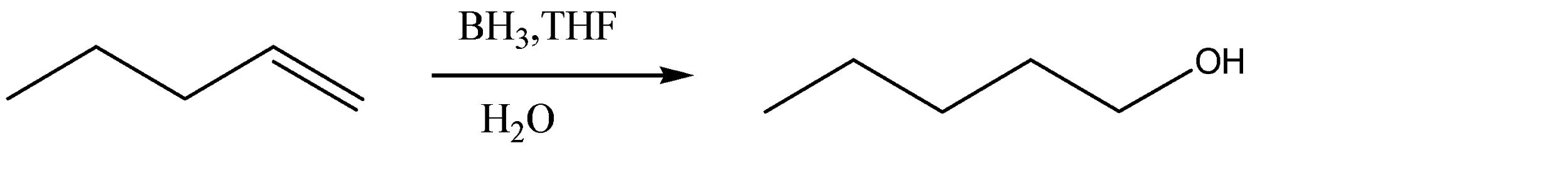
1.The given alcohol is formed at the terminal carbon at the alkane. Hence the pi bond of the alkene from which it is formed also exists in the last two carbon atoms. The intermediate structure formed due to the shifting of the pi bond to the terminal carbon would provide a site for linking the \[ - OH\] group. We can use hydroboration – oxidation for converting this alkene to alcohol.
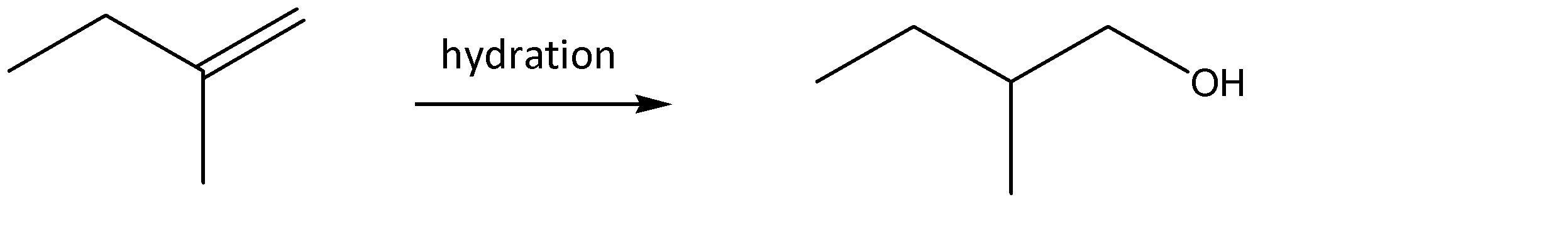
2.The given alcohol is formed at the terminal carbon where the pre – terminal carbon is a tertiary carbon atom. The intermediate structure formed due to the shifting of the pi bond to the terminal carbon would provide a site for linking the \[ - OH\] group. Hence the given alcohol can be formed by the following reaction:
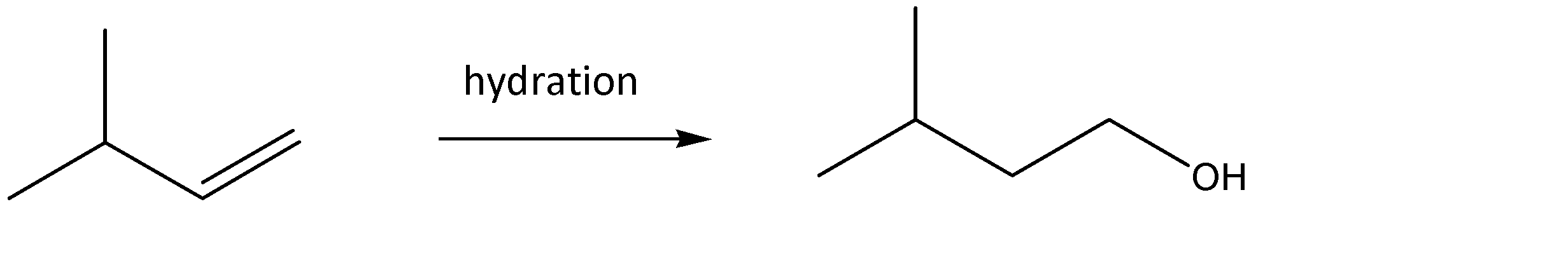
3.Similar to the first case, the alcoholic group is formed at the terminal carbon. The intermediate structure formed due to the shifting of the pi bond to the terminal carbon would provide a site for linking the \[ - OH\] group. The hydration of the corresponding alkene would take place by Anti Marchenko’s Rule. Hence, the corresponding reaction can be given as:
4.In the fourth option however, the carbon atom attached to the alcoholic group is a quaternary carbon atom. Formation of alkenes is not possible on quaternary carbons.
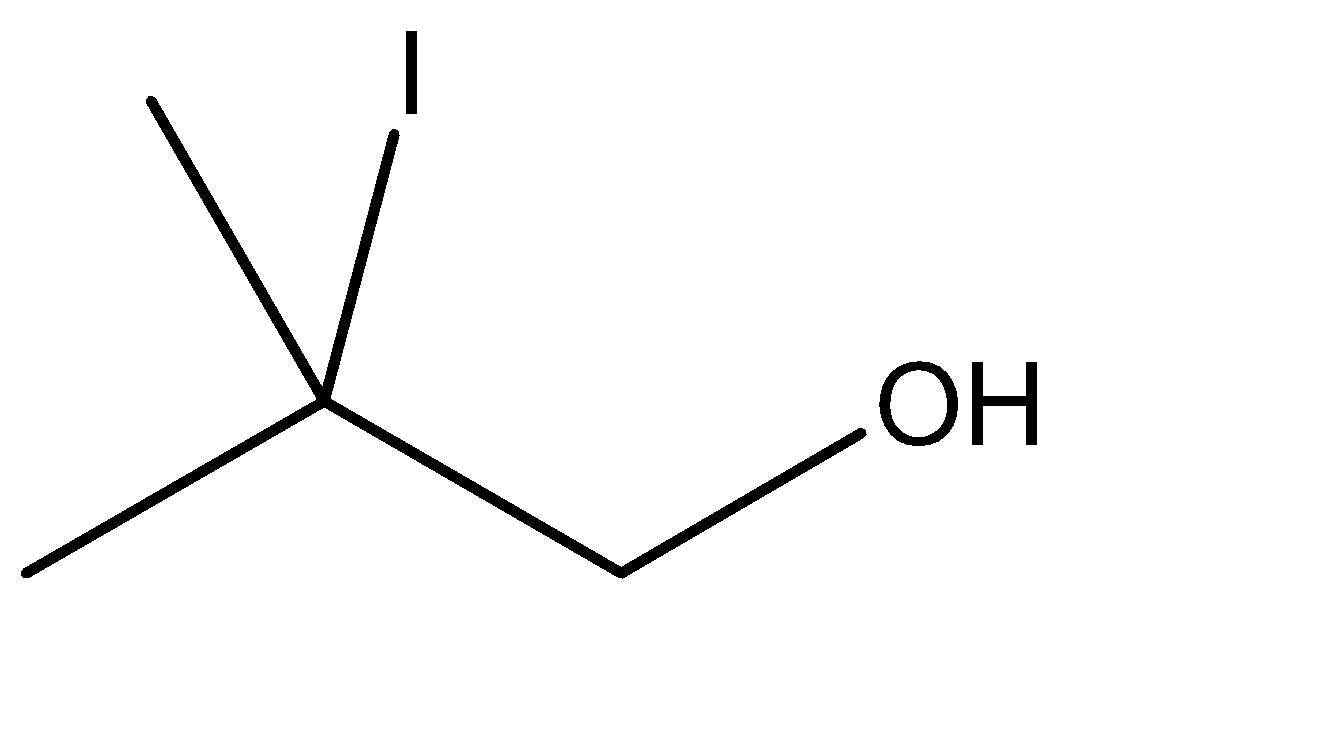
There is no scope for the formation of an intermediate and hence, there is no available site for linking the \[ - OH\] group.
Hence, Option D is the correct option.
Note: Anti-Markovnikov rule describes the Regio - chemistry where the substituent is bonded to a less substituted carbon, rather than the more substituted carbon. This rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
Complete Step-by-Step Answer:
Let us discuss the options one by one to discuss each case separately.
1.The given alcohol is formed at the terminal carbon at the alkane. Hence the pi bond of the alkene from which it is formed also exists in the last two carbon atoms. The intermediate structure formed due to the shifting of the pi bond to the terminal carbon would provide a site for linking the \[ - OH\] group. We can use hydroboration – oxidation for converting this alkene to alcohol.

2.The given alcohol is formed at the terminal carbon where the pre – terminal carbon is a tertiary carbon atom. The intermediate structure formed due to the shifting of the pi bond to the terminal carbon would provide a site for linking the \[ - OH\] group. Hence the given alcohol can be formed by the following reaction:

3.Similar to the first case, the alcoholic group is formed at the terminal carbon. The intermediate structure formed due to the shifting of the pi bond to the terminal carbon would provide a site for linking the \[ - OH\] group. The hydration of the corresponding alkene would take place by Anti Marchenko’s Rule. Hence, the corresponding reaction can be given as:

4.In the fourth option however, the carbon atom attached to the alcoholic group is a quaternary carbon atom. Formation of alkenes is not possible on quaternary carbons.

There is no scope for the formation of an intermediate and hence, there is no available site for linking the \[ - OH\] group.
Hence, Option D is the correct option.
Note: Anti-Markovnikov rule describes the Regio - chemistry where the substituent is bonded to a less substituted carbon, rather than the more substituted carbon. This rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




