
Which of the following acids has the property of flexibility?
A)
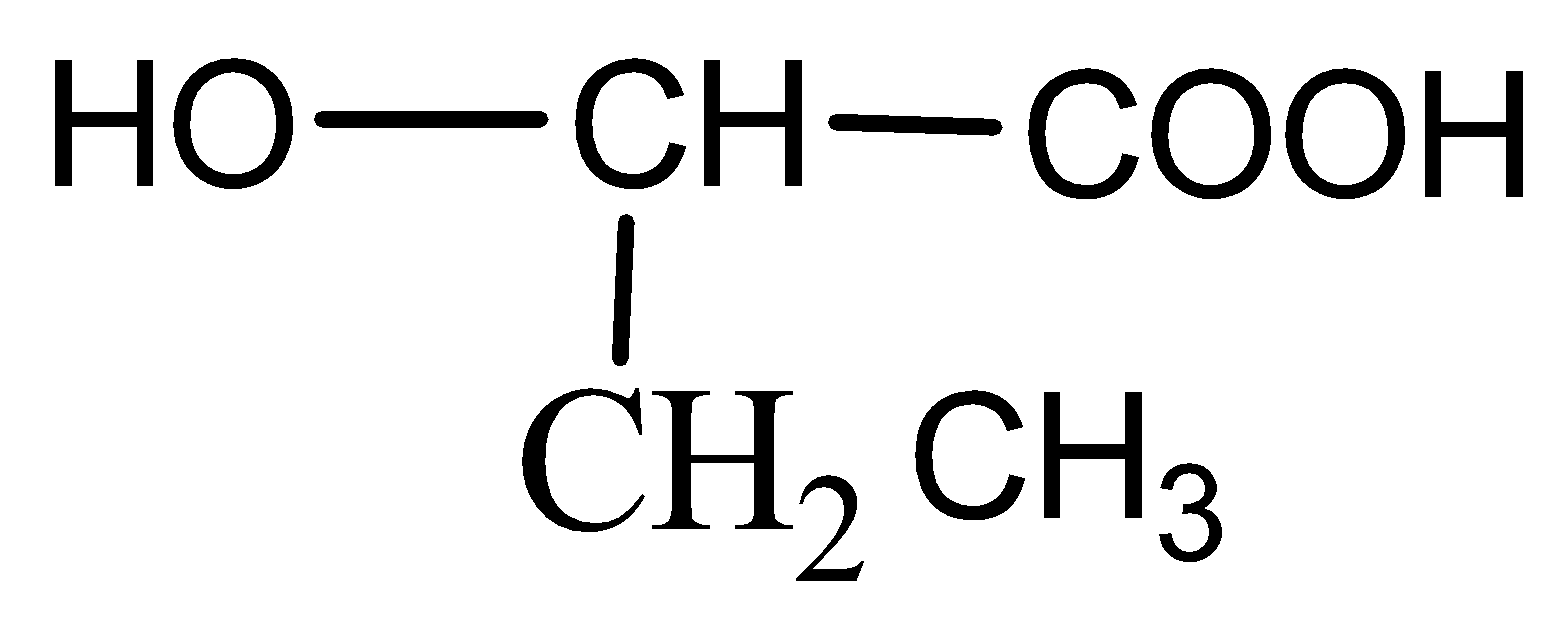
B)
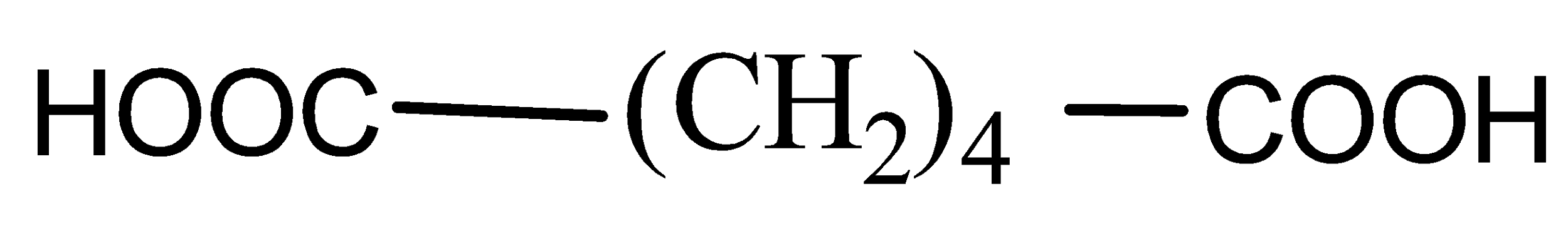
C)
D)
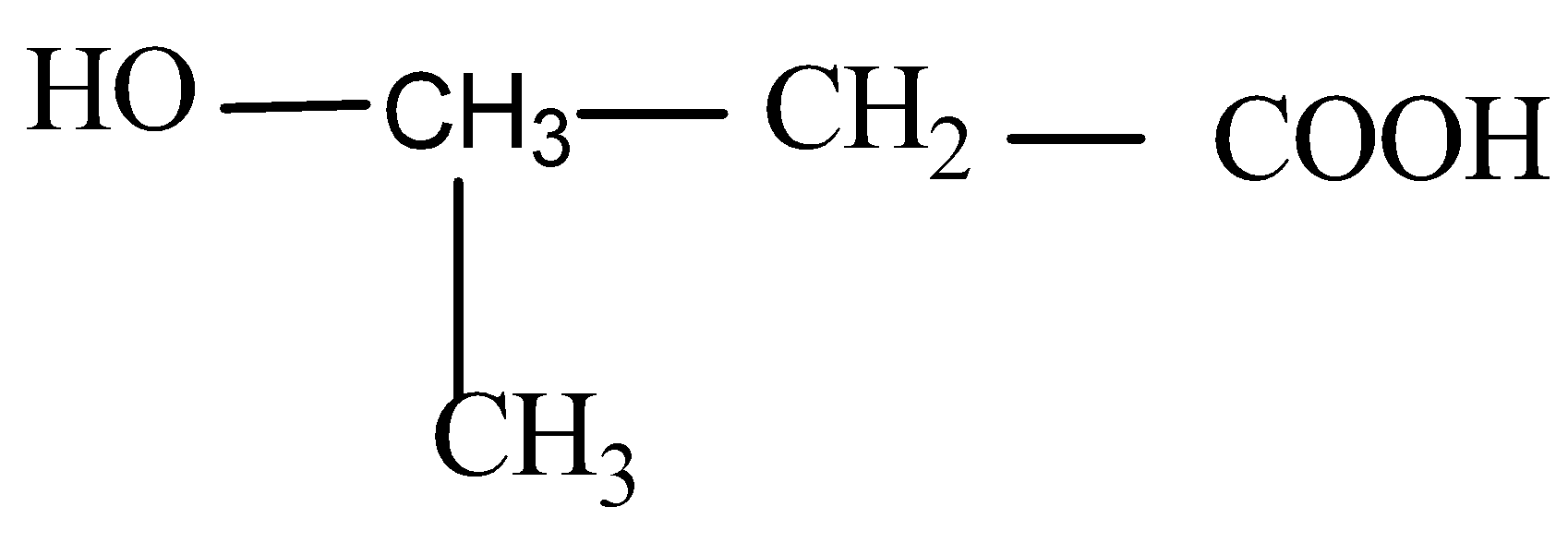
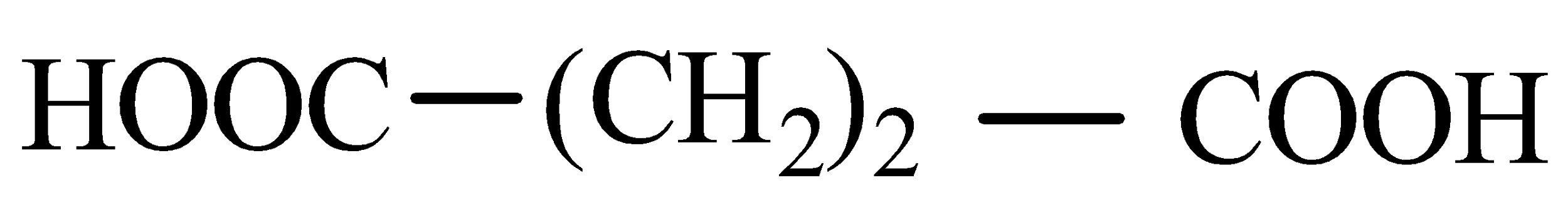
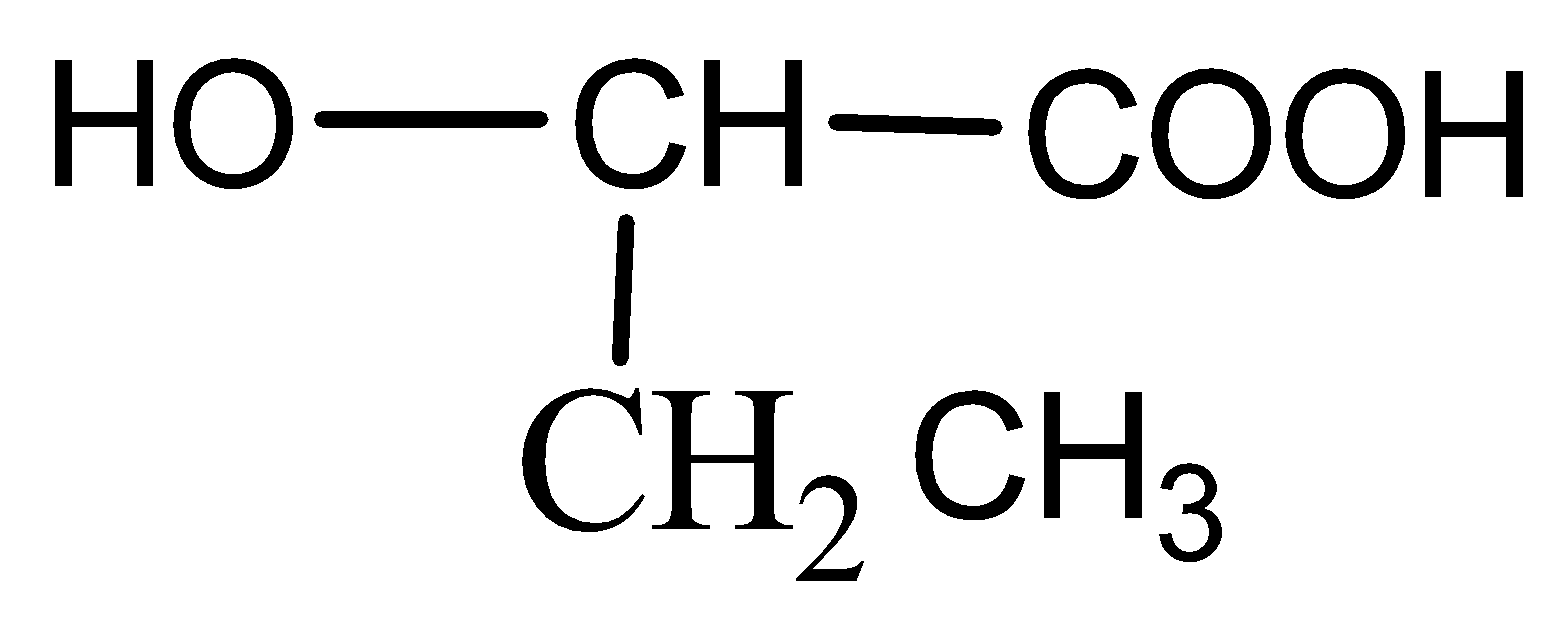
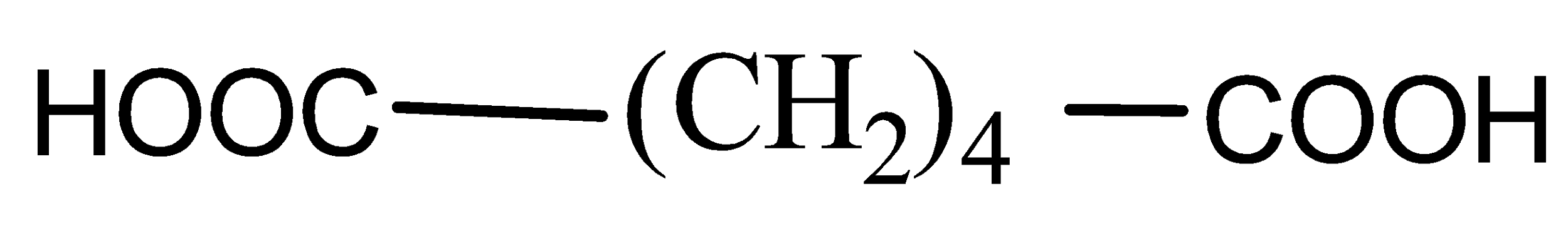
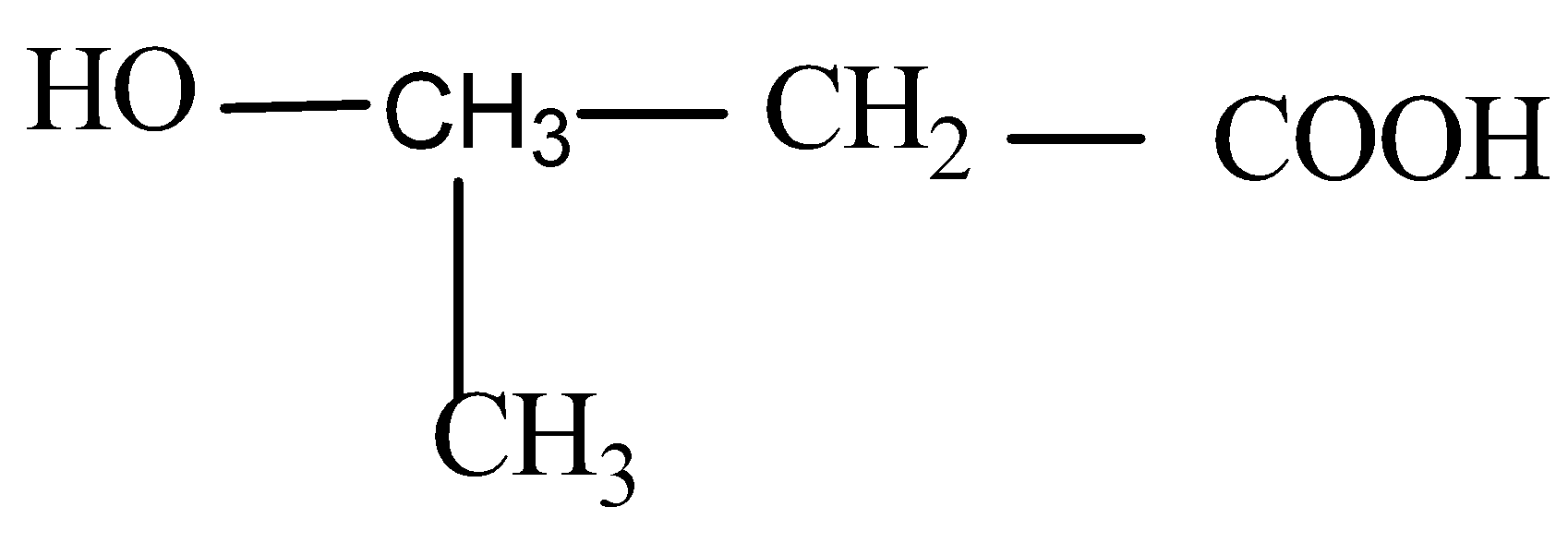
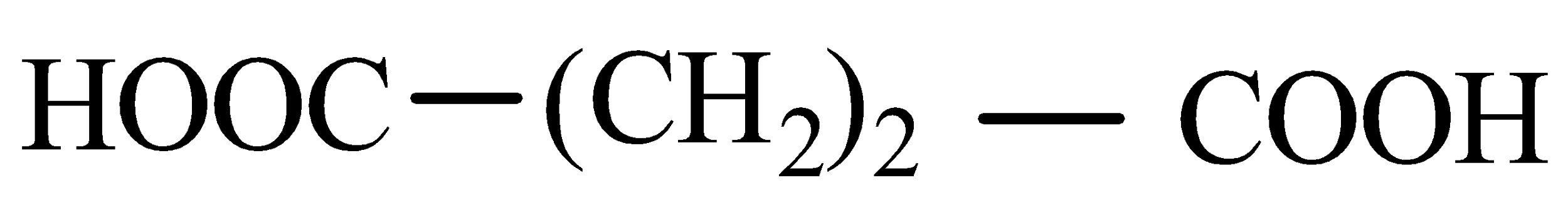
Answer
526.8k+ views
Hint: Generally, polymers are flexible in nature. Polymers are basically large molecules made up of a single repeating unit. Flexibility is mainly how much a bond can move as the polymer chains are constantly vibrating or rotating. If the thermal energy is more than more the polymers will move to give more flexibility.
Complete step by step solution:
Now when it comes to deciding which is more flexible, certain physical properties are responsible for it. The first is long-chain polymers, the longer the chain the stronger they are in nature. The flexibility of any polymer basically depends on the groups present in it. For example, the benzene ring does not provide flexibility but $C{H_2}$ does provide it. More number of oxygen atoms present also decreases the flexibility. Hence, we can easily eliminate options C and D. Now, as the degree of polymers increases the side chains become more flexible because they can rotate easily so the conclusion is if we have a longer side chain the polymers get more flexible. So the polymer $3 - 3 - hydroxybutyric{\text{ acid }}$ has property of flexibility
The structure is like this:
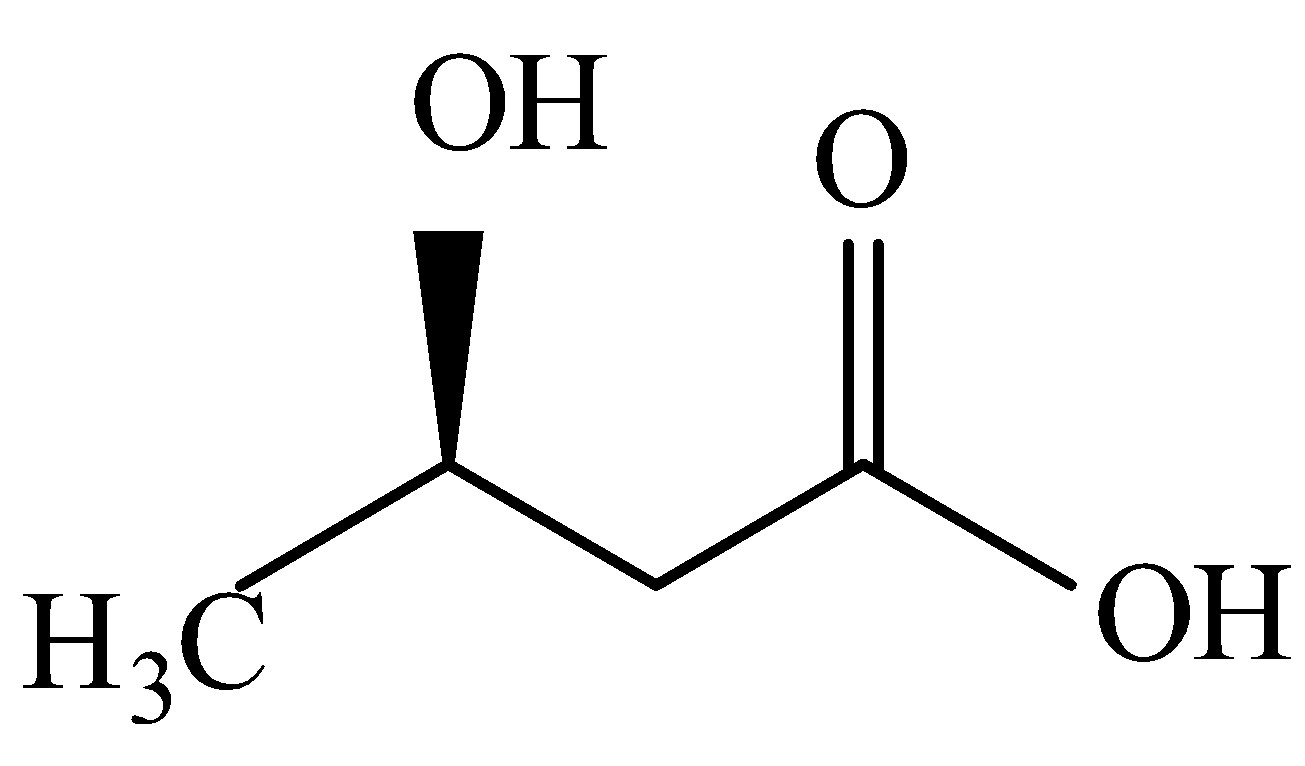
Hence, the correct answer is (C).
Note:
Flexibility increases with increase in molecular weight up to a certain point, Higher molecular weight results in more entanglement. The groups which are responsible for decreasing the flexibility are known as stiffening groups.
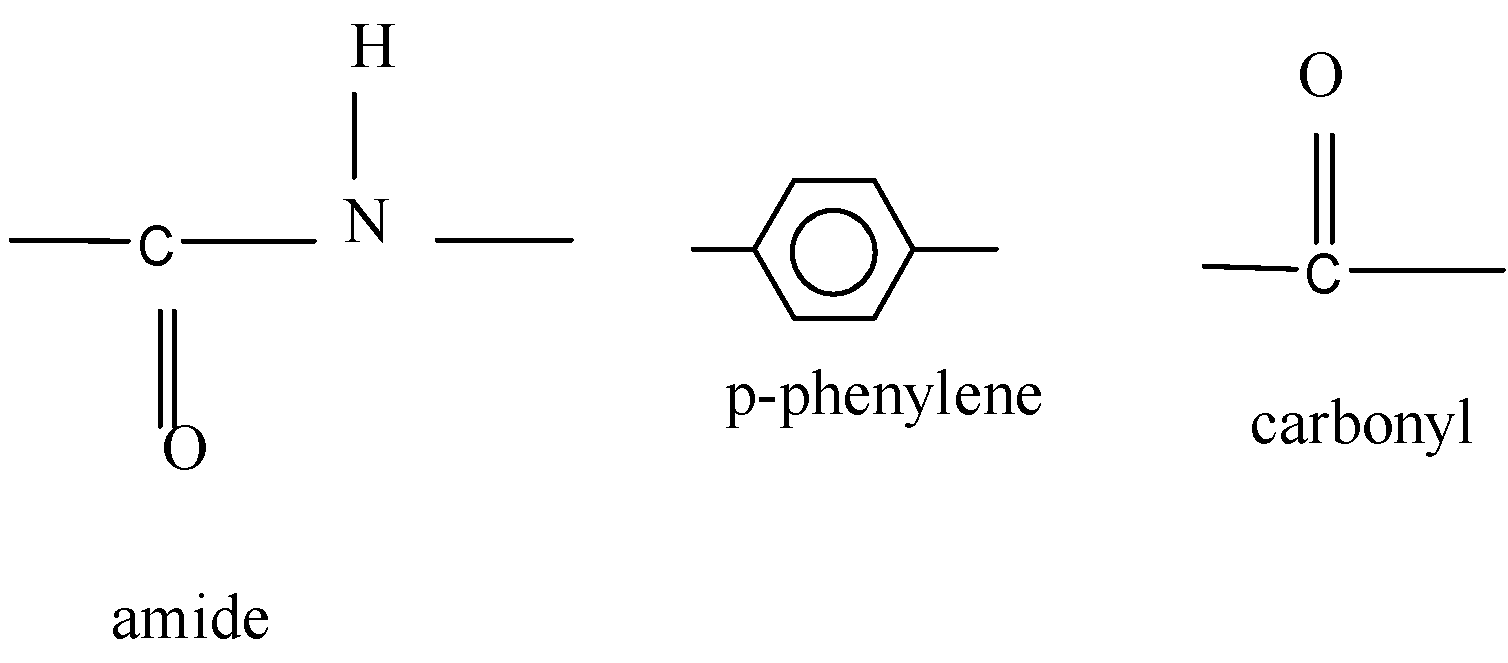
Here are few examples of it
Complete step by step solution:
Now when it comes to deciding which is more flexible, certain physical properties are responsible for it. The first is long-chain polymers, the longer the chain the stronger they are in nature. The flexibility of any polymer basically depends on the groups present in it. For example, the benzene ring does not provide flexibility but $C{H_2}$ does provide it. More number of oxygen atoms present also decreases the flexibility. Hence, we can easily eliminate options C and D. Now, as the degree of polymers increases the side chains become more flexible because they can rotate easily so the conclusion is if we have a longer side chain the polymers get more flexible. So the polymer $3 - 3 - hydroxybutyric{\text{ acid }}$ has property of flexibility
The structure is like this:

Hence, the correct answer is (C).
Note:
Flexibility increases with increase in molecular weight up to a certain point, Higher molecular weight results in more entanglement. The groups which are responsible for decreasing the flexibility are known as stiffening groups.
Here are few examples of it

Recently Updated Pages
Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




