
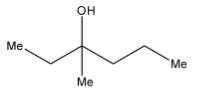
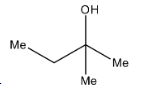
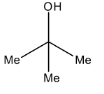
Which of the following ${{3}^{\circ }}$ alcohol does propyl ester give during reaction with EtMgBr?
A.
B.
C.
D.




Answer
533.7k+ views
Hint: Propyl ester means one part of the ester functional group is Propyl in which there are 3 carbon atoms whose formula is ${{C}_{3}}{{H}_{7}}$ and the other part will be R (an alkyl group). EtMgBr is a Grignard reagent, when this reacts with carbonyl group then there will be the addition of alkyl group and formation of alcohols.
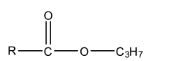
Complete answer: Propyl ester means one part of the ester functional group is Propyl in which there are 3 carbon atoms whose formula is ${{C}_{3}}{{H}_{7}}$ and the other part will be R (an alkyl group). The formula of the compound will be:
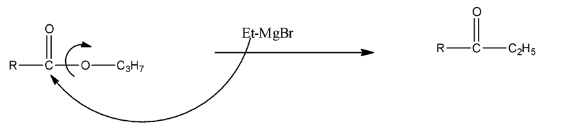
EtMgBr is a Grignard reagent, in which the MgBr is the positive part and the ethyl group is the negative part. When the compound reacts with EtMgBr, then the leaving group in the propyl ester, i.e., $-O{{C}_{3}}{{H}_{7}}$ will be replaced with the ethyl group of the Grignard reagent. This will form alcohol. The reaction is given below:
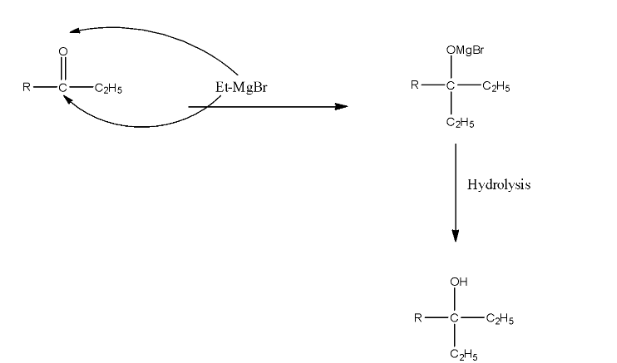
The formed compound will again react with the Grignard reagent because the formed compound is a ketone. There will be the addition of ethyl groups on the carbon atom and MgBr will be added on the Oxygen atom. Now, this compound on hydrolysis will form ${{3}^{\circ }}$ alcohol. The reaction is given below:
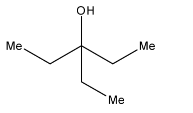
As we can see that there are two ethyl groups in the product, so this corresponds to the structure in option (a).
Therefore, the correct answer is an option (a).
Note: When the Grignard reagent reacts with any ester or any ketone then the final product will be ${{3}^{\circ }}$ alcohol, and if it reacts with aldehyde other than formaldehyde we get ${{2}^{\circ }}$ alcohol.
Complete answer: Propyl ester means one part of the ester functional group is Propyl in which there are 3 carbon atoms whose formula is ${{C}_{3}}{{H}_{7}}$ and the other part will be R (an alkyl group). The formula of the compound will be:
EtMgBr is a Grignard reagent, in which the MgBr is the positive part and the ethyl group is the negative part. When the compound reacts with EtMgBr, then the leaving group in the propyl ester, i.e., $-O{{C}_{3}}{{H}_{7}}$ will be replaced with the ethyl group of the Grignard reagent. This will form alcohol. The reaction is given below:

The formed compound will again react with the Grignard reagent because the formed compound is a ketone. There will be the addition of ethyl groups on the carbon atom and MgBr will be added on the Oxygen atom. Now, this compound on hydrolysis will form ${{3}^{\circ }}$ alcohol. The reaction is given below:

As we can see that there are two ethyl groups in the product, so this corresponds to the structure in option (a).

Therefore, the correct answer is an option (a).
Note: When the Grignard reagent reacts with any ester or any ketone then the final product will be ${{3}^{\circ }}$ alcohol, and if it reacts with aldehyde other than formaldehyde we get ${{2}^{\circ }}$ alcohol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




